Top Manufacturing Trends in China for Physiotherapy Devices 2025
- Top Manufacturing Trends in China for Physiotherapy Devices 2025
- Introduction — China medical device manufacturers and physiotherapy
- 1. Regulatory maturity and quality-first manufacturing
- Stricter NMPA oversight is raising quality bars
- Impact on China medical device manufacturers
- 2. Automation, smart factories and Industry 4.0
- Higher automation yields consistent, scalable output
- Digital twins and predictive maintenance
- 3. Embedded software, AI and connected rehabilitation
- Smart physiotherapy devices with onboard intelligence
- Tele-rehab and cloud connectivity
- 4. Growth of portable and home-use physiotherapy devices
- Demand shifts from clinics to homes
- Design for usability and service
- 5. Clinical evidence, outcomes data and reimbursement focus
- Evidence-based product positioning
- Role of data in payer discussions
- 6. Supply chain resilience and localized component sourcing
- Reducing dependency and improving lead times
- Strategic inventory and dual-sourcing
- 7. Additive manufacturing and rapid prototyping
- Faster iteration for custom and low-volume parts
- Tooling reductions and small-batch flexibility
- 8. Sustainability and green manufacturing
- Environmental responsibility is becoming a buyers’ criterion
- Regulatory and market incentives
- 9. Service, training and value-added offerings
- After-sales support complements product sales
- Localization of service teams
- 10. Competitive landscape: OEM, ODM and branded exports
- From contract manufacturing to branded innovation
- Partnerships and M&A
- Comparison of Key Trends and Their Manufacturing Impact (2025)
- How buyers should evaluate china medical device manufacturers in 2025
- Checklist for procurement teams
- Questions to ask suppliers
- Why international partners continue to work with China medical device manufacturers
- Cost-effective scaling combined with improved quality
- Ability to co-develop and localize products
- Case focus: Longest Medical — strengths and product advantages
- About Longest — credibility and positioning
- Longest's manufacturing and clinical strengths
- Product-by-product advantages
- focused shockwave therapy machine
- electrical muscle stimulation machine
- air relax compression
- active passive trainer
- compression therapy machine
- dvt medical device
- lymphatic massage device & Pressotherapy machine
- Why clinics choose Longest
- FAQ — Frequently asked questions about manufacturing trends and procurement
- Q1: Are china medical device manufacturers meeting international quality standards?
- Q2: How important is clinical evidence when sourcing physiotherapy devices?
- Q3: Can I get reliable after-sales support from China-based suppliers?
- Q4: What are the main risks when partnering with new manufacturers?
- Q5: How will trends in 2025 affect procurement costs?
- Q6: How to choose between OEM/ODM partners and buying branded products?
- Conclusion — positioning for success with china medical device manufacturers
- Summary and buyer guidance
Top Manufacturing Trends in China for Physiotherapy Devices 2025
Introduction — China medical device manufacturers and physiotherapy
China medical device manufacturers continue to evolve rapidly, moving from high-volume, low-cost OEM production to technology-driven, quality-focused suppliers for physiotherapy devices. In 2025, manufacturers are balancing regulatory compliance, digital innovation, and global market demands to serve rehabilitation clinics, sports medicine, homecare, and medical aesthetics.
1. Regulatory maturity and quality-first manufacturing
Stricter NMPA oversight is raising quality bars
China's National Medical Products Administration (NMPA) reforms over recent years have pushed manufacturers to strengthen quality management, clinical evidence, and post-market surveillance. For physiotherapy device makers, this means upgraded design controls, expanded clinical validation, and closer conformity with international standards such as ISO 13485 and IEC 60601.
Impact on China medical device manufacturers
Because of regulatory tightening, many China medical device manufacturers are investing in certified quality systems and clinical studies to maintain access to domestic and export markets. This has driven consolidation: smaller suppliers that cannot meet new standards are being acquired or replaced by stronger OEM/ODM partners.
2. Automation, smart factories and Industry 4.0
Higher automation yields consistent, scalable output
To meet demand while ensuring consistent device quality, physiotherapy equipment makers are automating manufacturing lines—robotic assembly, automated testing and vision inspection are increasingly common. Automation reduces human error and supports traceability, which is critical for regulatory dossiers and recalls management.
Digital twins and predictive maintenance
Leading manufacturers use digital twins and IoT-enabled machinery to monitor production health. Predictive maintenance lowers downtime and helps China medical device manufacturers keep lead times short while preserving manufacturing quality.
3. Embedded software, AI and connected rehabilitation
Smart physiotherapy devices with onboard intelligence
Physiotherapy devices increasingly include embedded software, AI-driven protocols, and data logging. From adaptive electrotherapy current profiles to AI-guided shockwave parameter selection, these features improve treatment personalization and can create new value chains based on software-as-a-feature or subscription services.
Tele-rehab and cloud connectivity
Tele-rehabilitation is accelerating device connectivity requirements. Manufacturers must provide secure firmware, HIPAA/GDPR-aware cloud interfaces and remote monitoring capabilities so clinicians can supervise home-based therapy sessions—this elevates the technical bar for China medical device manufacturers exporting to regulated markets.
4. Growth of portable and home-use physiotherapy devices
Demand shifts from clinics to homes
An aging population and preference for convenient, non-invasive care drive demand for portable electrotherapy units, compact shockwave devices, and wearable stimulators. China medical device manufacturers are expanding R&D into user-friendly, battery-powered devices with simple UIs and safety interlocks to address homecare adoption.
Design for usability and service
Manufacturers are investing in ergonomic product design, multilingual instructions, remote diagnostics, and modular repairability—features that reduce total cost of ownership for clinics and make home devices safer and easier to adopt.
5. Clinical evidence, outcomes data and reimbursement focus
Evidence-based product positioning
To win tenders and reimbursement, device makers must support clinical efficacy claims with well-structured clinical trials and real-world outcome data. Physiotherapy manufacturers in China are partnering with hospitals and research centers to collect meaningful outcomes that support device adoption and market access abroad.
Role of data in payer discussions
Outcomes data and health economic analyses are increasingly used to demonstrate cost-effectiveness—this is particularly important for chronic rehabilitation therapies where payers require proof of long-term benefit.
6. Supply chain resilience and localized component sourcing
Reducing dependency and improving lead times
Recent global disruptions have accelerated efforts to localize critical components and multi-sourcing strategies. China medical device manufacturers are working with domestic electronics, motor and consumable suppliers to shorten supply chains and reduce exposure to single-country risks.
Strategic inventory and dual-sourcing
Manufacturers are adopting buffer stock strategies and qualifying alternate suppliers to maintain production continuity for high-demand physiotherapy lines such as shockwave generators and compression therapy systems.
7. Additive manufacturing and rapid prototyping
Faster iteration for custom and low-volume parts
3D printing is used for rapid prototyping and producing patient-specific fittings, ergonomic housings, and some polymer components. This capability accelerates design cycles and supports custom accessories for rehabilitation devices.
Tooling reductions and small-batch flexibility
Additive manufacturing reduces tooling lead times and enables agile small-batch production—advantageous for specialized physiotherapy accessories and initial market tests.
8. Sustainability and green manufacturing
Environmental responsibility is becoming a buyers’ criterion
Hospitals and large clinic chains increasingly evaluate suppliers on sustainability. China medical device manufacturers are adopting recyclable packaging, energy-efficient production, and greener material choices to win procurement contracts and align with global ESG expectations.
Regulatory and market incentives
Sustainability initiatives are often linked to long-term cost savings and regulatory favorability, prompting manufacturers to disclose carbon intensity and material sourcing in supplier assessments.
9. Service, training and value-added offerings
After-sales support complements product sales
Rather than purely selling devices, successful manufacturers provide training, remote service, extended warranties, and outcome-monitoring platforms. For physiotherapy devices, clinical training and therapy protocols are key differentiators when hospitals choose a supplier.
Localization of service teams
To support global customers, many China medical device manufacturers now maintain regional service centers and local-language clinical educators to reduce barriers to adoption and ensure proper therapy delivery.
10. Competitive landscape: OEM, ODM and branded exports
From contract manufacturing to branded innovation
Many China medical device manufacturers have moved up the value chain—shifting from OEM/ODM roles to marketing their own brands internationally. This transition is supported by stronger IP practices, patent filings, and enhanced clinical credibility.
Partnerships and M&A
Strategic partnerships, licensing deals, and acquisitions help accelerate technology adoption—companies seeking fast entry into physiotherapy segments often partner with local innovators in China for manufacturing scale and cost efficiency.
Comparison of Key Trends and Their Manufacturing Impact (2025)
| Trend | Primary Manufacturing Impact | Adoption Timeline |
|---|---|---|
| Regulatory & Quality Upgrades | Higher QA systems, clinical testing, supplier audits | Already high; ongoing |
| Automation & Smart Factories | Consistent output, traceability, lower defect rates | Medium — expanding rapidly |
| AI & Connected Devices | Software development, cybersecurity, cloud ops | Accelerating — near term |
| Homecare & Portables | Battery tech, safety features, user UX | High — immediate |
| Sustainability | Material choices, packaging, energy use | Growing — next 3–5 years |
How buyers should evaluate china medical device manufacturers in 2025
Checklist for procurement teams
When sourcing physiotherapy devices, evaluate manufacturers on: regulatory certifications (NMPA, CE, ISO 13485), clinical evidence and references, software/cybersecurity practices, after-sales capability, local service footprint, and supply chain robustness. Cost remains important but should be weighed against quality and lifecycle support.
Questions to ask suppliers
Key procurement questions include: can you provide clinical study data for the device? What is your typical lead time and alternative sourcing plan? Do you support remote diagnostics and software updates? What warranty and training services do you offer?
Why international partners continue to work with China medical device manufacturers
Cost-effective scaling combined with improved quality
China offers a mature manufacturing ecosystem—precision plastics, electronics, motors and assembly expertise—combined with increasingly strict quality systems. This combination enables partners to scale production commercially while meeting tougher regulatory and clinical demands.
Ability to co-develop and localize products
Many China medical device manufacturers now offer engineering, regulatory and clinical support for co-development, reducing time-to-market for new physiotherapy innovations.
Case focus: Longest Medical — strengths and product advantages
About Longest — credibility and positioning
Founded in 2000, Longest Medical is a leading global rehabilitation and aesthetic solutions company focusing on non-invasive medical solutions. Longest's product lines include shockwave therapy, compression therapy, electrotherapy, electrostatic oscillation therapy, cryotherapy, ultrasound therapy, and active-passive trainers. These products support physical therapy, neurological rehabilitation, postoperative recovery, veterinary care, and medical aesthetics.
Longest's manufacturing and clinical strengths
Longest benefits from over two decades of experience, combining established manufacturing processes with clinical partnerships to validate outcomes. Their global reach and comprehensive product portfolio make them a reliable partner for clinics seeking an integrated rehabilitation solution.
Product-by-product advantages
shockwave therapy machine: Longest's shockwave devices are designed for consistent energy output, easy parameter control, and clinically oriented protocols useful in musculoskeletal rehabilitation.
focused shockwave therapy machine
Focused shockwave devices concentrate energy deeper into tissues—Longest offers focused models with precise targeting, adjustable focal depth and documented protocols suitable for tendon and deep tissue therapy.
electrical muscle stimulation machine
Longest's EMS units combine multiple waveform options, safety limits and program presets for strength training, neuromuscular re-education, and pain management—designed for both clinic and home use.
air relax compression
Air Relax compression systems deliver sequential pneumatic compression for lymphedema management and recovery. Longest emphasizes adjustable pressure profiles, multi-chamber garments, and safety monitoring to ensure therapeutic efficacy.
active passive trainer
The active-passive trainers from Longest support early mobilization and continuous passive motion—useful after orthopedic procedures. Key advantages include programmable range-of-motion, smooth actuation and clinician-friendly interfaces.
compression therapy machine
Compression therapy devices are engineered to support venous return and edema control. Longest provides configurable cycles and clinical-grade garments built to sustained-use standards.
dvt medical device
Devices aimed at DVT prevention incorporate timed pneumatic compression, user safety interlocks, and clinical validation for thromboprophylaxis—Longest's DVT solutions meet common hospital requirements for prevention protocols.
lymphatic massage device & Pressotherapy machine
Longest's lymphatic massage and pressotherapy systems focus on patient comfort, customizable programs, and durable garments. These features improve patient adherence and clinical outcomes in lymphatic drainage and aesthetics applications.
Why clinics choose Longest
Clinics value Longest for its broad therapy portfolio (allowing package solutions), long operational history, and focus on non-invasive, evidence-informed technologies. Their mix of clinical training, technical support and product reliability fits the increasing expectations of buyers influenced by modern regulatory and outcomes requirements.
FAQ — Frequently asked questions about manufacturing trends and procurement
Q1: Are china medical device manufacturers meeting international quality standards?
A1: Many leading manufacturers in China hold ISO 13485 certification and can meet CE, NMPA and other market-specific requirements. Buyers should verify certificates, audit reports and clinical data before procurement.
Q2: How important is clinical evidence when sourcing physiotherapy devices?
A2: Clinical evidence is crucial—hospitals and payers increasingly require clinical outcomes and safety data to support product selection and reimbursement. Manufacturers that provide peer-reviewed studies or real-world outcome registries are preferred.
Q3: Can I get reliable after-sales support from China-based suppliers?
A3: Top-tier China medical device manufacturers maintain regional service centers, certified trainers and remote support systems. Confirm local service arrangements, spare-parts availability and warranty terms during vendor evaluation.
Q4: What are the main risks when partnering with new manufacturers?
A4: Key risks include regulatory non-compliance, inconsistent quality, weak clinical support, and fragile supply chains. Mitigate these by performing due diligence: factory audits, requesting clinical references, and checking supplier financials and multi-sourcing strategies.
Q5: How will trends in 2025 affect procurement costs?
A5: Initial costs may rise due to investments in quality systems, automation and clinical studies. However, lifecycle costs often improve via reduced defects, better outcomes, lower service overhead, and economies of scale for software-enabled services.
Q6: How to choose between OEM/ODM partners and buying branded products?
A6: Choose OEM/ODM for custom cost-effective volume products or fast iterations; choose branded suppliers if clinical evidence, long-term support and warranty-backed performance are priorities. Many buyers find hybrid approaches effective—co-develop a product with a reputable manufacturer.
Conclusion — positioning for success with china medical device manufacturers
Summary and buyer guidance
In 2025, physiotherapy device manufacturing in China is characterized by higher regulatory expectations, stronger automation, AI-enabled products, growth in homecare devices, and a rising focus on sustainability and clinical evidence. Buyers should prioritize suppliers that demonstrate QA rigor, service capability, clinical validation, and secure software practices. Established players such as Longest Medical exemplify the evolution of China medical device manufacturers—offering broad non-invasive therapy portfolios, clinical partnerships and global support suitable for modern rehabilitation needs.
Compression Machine for Legs Benefits
Top 10 Electrotherapy Devices for Clinic Use (2025)
Active Passive Trainer Cost in Spain: Buyer’s Guide for Clinics and Suppliers
Active Passive Trainer Cost in UK: Prices, Buying Guide & Where to Buy
LGT - 2360S
Why is the PowerOsci LGT - 2360S recommended for purchase?
PowerOsci LGT-2360S is a handheld electrostatic oscillation device that enables easy and efficient whole-body treatment. It is durable and has a wide range of applications. It is FDA-approved and comes with excellent after-sales service. It is the top choice for physiotherapy and beauty practitioners to reduce workload, improve efficiency, and provide a better experience for patients.
2500s plus physical therapy version
Does your product come with a warranty?
Yes, we're committed to delivering quality products and excellent after-sales service to enhance your overall experience.
LGT-2320BE
Are there any side effects of using Electrical Stimulation Machine for aesthetic purposes?
Side effects are rare and minor. They are generally short-lived and resolve on their own. Consult a professional before treatment, especially if you have any concerns or underlying medical conditions.
LGT-2202DVT
How long should a DVT pump be used?
The duration of DVT pump usage depends on the individual's condition. In some cases, they may be used during hospitalization and then discontinued once the risk of thrombosis decreases. In other instances, individuals may be instructed to continue using the pump at home for a specific period or until they recover fully.
LGT-2510B - Beauty Edition
How does PowerShocker LGT - 2510B assist with patient management?
It can save patients' information and treatment records. This gives caregivers access to valuable patient data, enabling them to make more informed decisions and achieve better treatment results.

Professional 12-Channel Low-Frequency Electrical Stimulation Machine LGT-2320SP
LGT-2320SP is an advanced electrical stimulation sports training station. It leads to multisite high-efficiency strength gain and realizes bilateral balanced development with motor point detection.
Specifically designed for sports medicine professionals, this device is ideal for the treatment and rehabilitation of sports-related injuries. It delivers precise electrical impulses to targeted areas of the body, promoting pain relief, muscle strengthening, and accelerated recovery.

Professional 12-Channel Low-Frequency Electrical Stimulation Machine LGT-2320BE
The LGT-2320BE is an advanced EMS machine, leveraging the power of low-frequency electrical stimulation therapy to achieve remarkable results in body sculpting.
It not only efficiently contours and tones the body but also actively promotes muscle relaxation, relieving tension and soreness. Moreover, it plays a significant role in enhancing skin elasticity, rejuvenating the skin's appearance and suppleness.
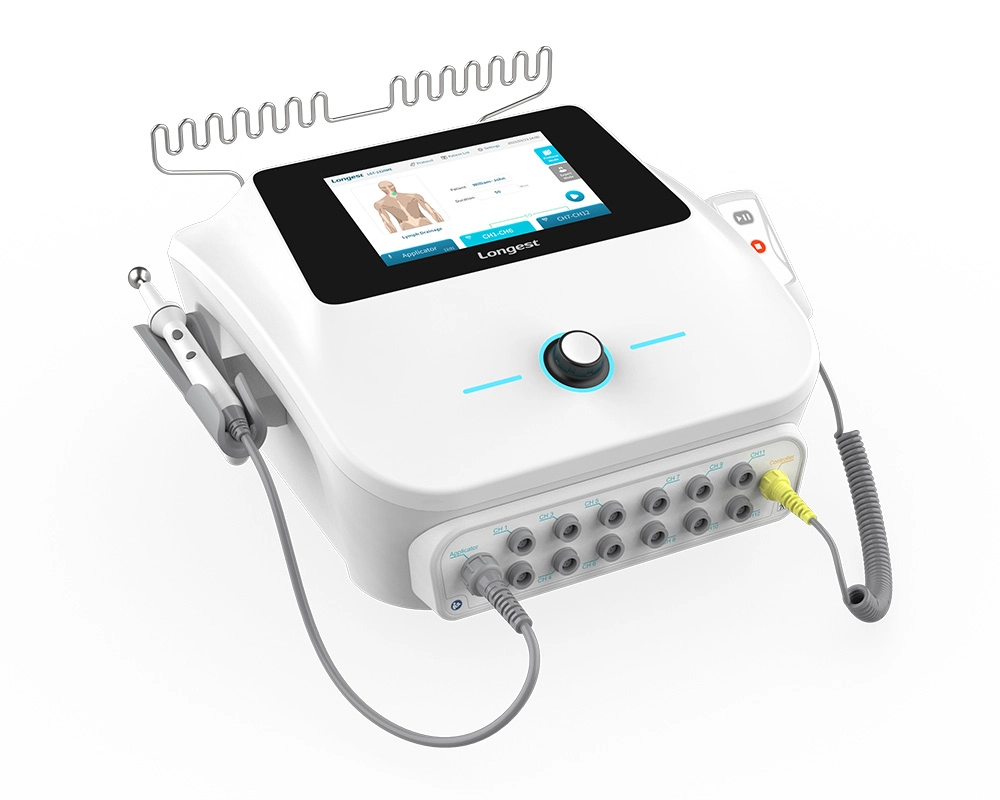
Neuromuscular Electrical Stimulation NMES Machine LGT-2320ME
The LGT-2320ME, a portable electro-stimulation therapy device, is mainly composed of the main unit, a hand switch, a pen electrode controller, and diverse types of electrodes.
It has the capacity to supply three channel groups (CH1-CH6, CH7-CH12, and the Applicator channel) with either TENS or NMES current. Specifically engineered for utilization in hospitals and clinics, this device effectively assists patients in reclaiming lost muscle strength and expedites recovery times, consequently enhancing the overall standard of patient care and bringing about more satisfactory rehabilitation outcomes.

Rehab Bike for Upper and Lower Limbs, Active-Passive Trainer (APT) RehaMoto LGT-5100D
RehaMoto controls the servo motor through the central processing unit and the biomechanical monitoring feedback system. Users can do passive, assisted, active, and constant-speed training by RehaMoto LGT-5100D Config I. The intelligent identification device realizes real - time monitoring of user training status and smooth conversion between different modes. Fully realize the best clinical training effect, and promote the recovery of users' motor function.
© 2025 Longest Medical. All Rights Reserved. Powered by gooeyun.






LongestMedical
LongestGloba
longest
guangzhou_longest
GzLongest