Maintenance, Cleaning and Calibration of Shockwave Devices
- Maintenance, Cleaning and Calibration of Shockwave Devices
- Why proper care of your shockwave therapy machine matters
- Daily pre-use checks for reliable operation
- Cleaning and disinfection after each patient
- Weekly and monthly care tasks to prevent wear
- Scheduled preventive maintenance and calibration
- Recommended maintenance schedule (practical table)
- Calibration specifics for shockwave devices
- How to validate device performance between calibrations
- Cleaning agents and infection control best practices
- Parts, consumables and inventory control
- Troubleshooting common issues with shockwave therapy machines
- Record-keeping and regulatory compliance
- Training and user competency
- When to contact the manufacturer or a certified service partner
- Comparing focused and radial shockwave machines: maintenance implications
- Cost-effective strategies to extend device life
- Selecting a service partner and warranty considerations
- Environmental and safety considerations
- Why documentation and E-E-A-T-aligned content matter for clinicians
- Longest Medical — company strengths and product overview
- Key Longest Medical products and advantages
- FAQ — Frequently asked questions about maintenance, cleaning and calibration
Maintenance, Cleaning and Calibration of Shockwave Devices
Why proper care of your shockwave therapy machine matters
Proper maintenance, cleaning and calibration of a shockwave therapy machine preserves clinical performance, reduces downtime, extends device life, and protects patients and staff. Shockwave devices deliver mechanical energy to tissues; if output drifts or transducers wear, clinical effectiveness and safety can be compromised. Routine care also helps clinics meet regulatory and infection-control expectations.
Daily pre-use checks for reliable operation
Before each treatment session, perform a daily checklist to verify the shockwave therapy machine is ready. Check power and battery levels (if portable), visual condition of the handpiece and cables, device self-test messages, emergency stop function, and applicator coupling. Confirm accessories, gel and single-use items are available and unopened. Document the check in the device log.
Cleaning and disinfection after each patient
After every patient, clean and disinfect the contact surfaces of the handpiece and any accessories that touch skin. Use manufacturer-recommended cleaning agents—many manufacturers endorse 70% isopropyl alcohol or hospital-grade disinfectants compatible with device materials. Avoid abrasive cleaners or solvents that may damage seals and plastics. For single-use items or disposable covers, follow single-use instructions and dispose of biohazard materials per local regulation.
Weekly and monthly care tasks to prevent wear
On a weekly or monthly basis, depending on throughput, perform deeper inspections: examine handpiece and cable insulation for cracks, verify applicator tip integrity, check mounting brackets and connectors, and clean air vents and cooling fans with low-pressure air to prevent overheating. Replace consumable applicator tips as recommended by the manufacturer or sooner if wear is visible. Verify software is up to date and clinical protocols are intact.
Scheduled preventive maintenance and calibration
Most manufacturers recommend formal preventive maintenance and calibration at least annually. Preventive maintenance typically includes internal inspections, replacement of wear parts, firmware updates, verification of electrical safety (per IEC 60601 series), and functional testing. Calibration ensures that acoustic output and energy delivery meet the manufacturer specifications. Because acoustic measurements require specialized equipment (hydrophones, calibrated sensors), calibration and acoustic output verification are commonly performed by the manufacturer or accredited service labs.
Recommended maintenance schedule (practical table)
Use this table as a practical baseline. Always follow the device-specific instructions for use (IFU) or the manufacturer service schedule.
| Task | Frequency | Who | Why |
|---|---|---|---|
| Pre-use visual & functional check | Every session | Operator/clinician | Detect visible damage, ensure safe operation |
| Surface cleaning & disinfection | After every patient | Operator/cleaning staff | Infection control and patient safety |
| Consumable/applicator inspection | Weekly / per high-use clinic monthly | Clinic staff | Prevent degraded energy transfer and tissue damage |
| Internal filter / fan cleaning | Monthly | Biomedical/clinic staff | Maintain cooling and prevent overheating |
| Software updates and data backup | Quarterly or as released | Clinic IT/biomed | Security, new features, and protocol fidelity |
| Full preventive maintenance & calibration | Annually (or as manufacturer specifies) | Manufacturer or certified service | Verify acoustic output and electrical safety |
Calibration specifics for shockwave devices
Calibration for a shockwave therapy machine often focuses on verifying energy output, pulse frequency and waveform characteristics. Accredited testing uses hydrophones, calibrated sensors, or manufacturer-specific test fixtures to measure output parameters. Because small changes in transducer wear or coupling can alter delivered energy, annual calibration is a common industry standard. Clinics that perform high-volume treatments may choose semi-annual service intervals. Always obtain a written calibration certificate and retain it for regulatory audits.
How to validate device performance between calibrations
Between formal calibrations, perform functional checks: track shot counts, log delivered energy settings during treatments, and monitor patient outcomes. If you observe a sudden change in patient response, unusual device noise, or visual defects on applicators, immediately stop use and arrange a service inspection. Some clinics use simple surrogate tests—such as measuring delivered pressure with a calibrated tactile or pressure sensor—for in-house trend monitoring, but these are not substitutes for accredited acoustic calibration.
Cleaning agents and infection control best practices
Select cleaning agents consistent with the device IFU. Commonly used disinfectants include 70% isopropyl alcohol, quaternary ammonium compounds, and hospital-grade surface disinfectants. Use soft, lint-free cloths; avoid high-pressure sprays that could drive liquids into connectors. For devices used on open wounds or compromised skin, use barrier methods and single-use applicator covers when available. Maintain a written infection-control protocol and train staff regularly.
Parts, consumables and inventory control
Keep an inventory of manufacturer-recommended consumables and spare parts—tips, applicator seals, O-rings, fuses, and single-use items. Using approved parts preserves performance and warranty coverage. Track usage rates and reorder before stockouts to avoid treatment delays. For portable units, maintain spare batteries and charging accessories.
Troubleshooting common issues with shockwave therapy machines
When a machine exhibits problems, use a stepwise approach: (1) verify the basics—power, fuses, cables, and connectors; (2) check software messages and event logs; (3) inspect applicator tips and seals for wear; (4) confirm correct coupling gel and technique; (5) consult the troubleshooting section of the IFU; (6) contact manufacturer technical support if the problem persists. Document all troubleshooting steps and device downtime in the service log.
Record-keeping and regulatory compliance
Maintain a device log that includes daily checks, cleaning records, firmware updates, incident reports, preventive maintenance, and calibration certificates. In many jurisdictions, such records are essential for compliance with medical device regulations and for credentialing in clinics. Accurate logs also support warranty claims and service planning.
Training and user competency
Ensure clinicians and technicians are trained in correct operation, coupling technique, infection control, and recognizing signs of device degradation. Manufacturer-provided training and competency assessments reduce misuse and extend device life. Include refresher training annually or when protocols are updated.
When to contact the manufacturer or a certified service partner
Contact the manufacturer or certified service provider if you suspect altered acoustic output, hear unusual internal noises, detect electrical faults, observe fluid ingress, or if the device displays persistent fault codes. Manufacturers can issue safety notices, provide parts and firmware updates, and supply calibration services that preserve device performance and regulatory compliance.
Comparing focused and radial shockwave machines: maintenance implications
Focused and radial shockwave therapy machines differ clinically and in maintenance concerns. Focused devices use focused acoustic energy through internal transducers and often require careful monitoring of transducer integrity and acoustic calibration. Radial devices use projectile-driven or compressed-air systems with different wear points such as seals and drive chambers. Below is a general comparison table to help maintenance planning.
| Characteristic | Focused Shockwave Machine | Radial Shockwave Machine |
|---|---|---|
| Clinical use | Deeper, more focal energy for targeted lesions | Superficial to mid-depth treatment, broader area |
| Maintenance focus | Transducer integrity, acoustic calibration | Mechanical drive parts, seals, projectile wear |
| Calibration frequency | Annual (or per manufacturer) | Annual (or per manufacturer); mechanical components may need more frequent inspection |
| Typical consumables | Applicator tips, coupling interfaces | Projectile or emitter modules, O-rings, tips |
Cost-effective strategies to extend device life
To maximize return on investment for your shockwave therapy machine: adhere to the IFU, schedule preventive maintenance, use manufacturer-approved consumables, train staff, and promptly repair minor faults before they escalate. Trend monitoring of shot counts and performance metrics helps predict when parts should be replaced rather than waiting for failure.
Selecting a service partner and warranty considerations
Choose service partners authorized by the device manufacturer. Authorized partners use approved parts and follow official procedures—important for warranty preservation. Review warranty terms for coverage of consumables versus core components, and confirm turnaround times for service to minimize clinical disruption.
Environmental and safety considerations
Store and operate the shockwave therapy machine within the manufacturer-specified temperature and humidity ranges. Protect the device from liquids and corrosive chemicals. Follow electrical safety protocols, including use of properly grounded outlets and avoiding extension cords that can introduce voltage drops. Dispose of consumables and batteries in accordance with local regulations.
Why documentation and E-E-A-T-aligned content matter for clinicians
Clear, evidence-aligned maintenance protocols support effective clinical use and institutional trust. Documentation that adheres to Expertise, Experience, Authoritativeness and Trustworthiness (E-E-A-T) helps clinical teams and procurement to justify service investments and ensures consistent patient care.
Longest Medical — company strengths and product overview
Founded in 2000, Longest Medical is a global rehabilitation and aesthetic solutions company focused on non-invasive medical technologies. Longest has built extensive experience in rehabilitation, physical therapy and medical aesthetics, offering a broad portfolio that supports clinics, hospitals and veterinary settings. Their advantage lies in integrated solutions that combine multiple therapy modalities, global service networks and a history of product development aligned with clinical needs.
Key Longest Medical products and advantages
Longest’s product range supports a comprehensive care pathway. Notable offerings include:
- Shockwave therapy machine / Focused shockwave therapy machine: Designed for musculoskeletal and aesthetic indications, with ergonomic handpieces, validated clinical protocols and manufacturer service support for calibration and consumables.
- Electrical muscle stimulation machine (EMS): For neuromuscular rehabilitation and functional recovery using programmable stimulation protocols.
- Air Relax compression / Compression therapy machine / Pressotherapy machine: For lymphatic drainage, circulation improvement and postoperative recovery with adjustable pressures and treatment programs.
- Active-passive trainer: Devices to assist motor relearning and active-passive rehabilitation exercises for neurological and orthopedic recovery.
- DVT medical device: Mechanical prophylaxis solutions to reduce deep vein thrombosis risk in perioperative and immobile patients.
- Lymphatic massage device: Designed to support lymphatic drainage therapies with validated programs and comfortable interfaces.
Advantages of Longest’s product line include broad modality coverage (so clinics can standardize procurement), manufacturer-backed training and service, and devices tailored for both clinical and aesthetic settings. For shockwave machines, Longest typically provides accessories, consumables and calibration support—the elements clinics need to maintain consistent performance and regulatory compliance.
FAQ — Frequently asked questions about maintenance, cleaning and calibration
Q: How often should I calibrate my shockwave therapy machine?
A: Annual calibration by the manufacturer or an accredited service lab is common. High-volume clinics may consider semi-annual checks. Always follow the manufacturer’s IFU.
Q: What disinfectants are safe for use on shockwave handpieces?
A: Use manufacturer-recommended disinfectants. Common choices include 70% isopropyl alcohol and approved hospital-grade cleaners. Avoid abrasive or solvent-based cleaners unless explicitly allowed.
Q: Can we perform in-house output testing?
A: Clinics can perform trend checks (shot counts, basic functional tests), but accredited acoustic measurements require specialized equipment and are typically performed by manufacturers or certified labs.
Q: How do I know when an applicator tip needs replacement?
A: Replace tips when you see visible wear, cracks, changes in coupling performance, or when recommended counts are exceeded. Degraded tips can reduce efficacy and increase patient discomfort.
Q: Does maintenance void my warranty?
A: Routine maintenance per IFU normally preserves warranty. Using unauthorized parts or unapproved repair services can void coverage—use authorized service partners.
Q: What records should my clinic keep?
A: Keep a device log with daily checks, cleaning records, software updates, incident reports, preventive maintenance and calibration certificates for audits and quality control.
Q: Who should perform electrical safety testing?
A: Qualified biomedical engineers or certified service technicians should perform electrical safety testing, typically according to IEC 60601 standards and local regulations.
Q: How can clinics minimize downtime during service?
A: Maintain spare consumables and, if budget allows, backup or rental units. Schedule preventive maintenance during low-volume periods and keep close coordination with authorized service partners.
Active Passive Trainer Cost in Miami: A Practical Buyer’s Guide for Clinics and Providers
Top 10 physio equipment Manufacturers and Supplier Brands
Wholesale electro-therapeutic devices manufacturer and supplier
Comprehensive Shockwave Therapy Machine Instructions for Optimal Use
LGT-2510B - Beauty Edition
Does your product come with a warranty?
Yes, we're committed to delivering quality products and excellent after-sales service to enhance your overall experience.
2510A-beauty
In which circumstances is AWT not suitable?
Pregnancy
Over major blood vessels and nerves
Having pacemakers or other implanted devices
Open wounds Taking oral anti - coagulants
Having a blood clotting disorder
Having received a Steroid injection within 6 weeks
Tumors are present at the treatment site
Having skin infection or abrasion at the treatment site
Electrostatic Oscillation
Is the operation complicated?
The operation is very simple, you can get started in just a few steps, and it comes with a detailed operation guide and video tutorial. Even non-professionals can learn quickly, which is very suitable for professionals and individuals to use at home.
Products
What international certifications do the company's products have?
The products have obtained international authoritative certifications such as CE, FDA, NRTL (North America), and GOST. They have also passed the MDSAP (ISO 13485:2016) certification, complying with the regulatory and standard requirements of multiple countries and regions.
LGT-2320SP
Is OEM/ODM service available?
Yes. Contact us for more details. We offer professional OEM/ODM services.

Professional 12-Channel Low-Frequency Electrical Stimulation Machine LGT-2320SP
LGT-2320SP is an advanced electrical stimulation sports training station. It leads to multisite high-efficiency strength gain and realizes bilateral balanced development with motor point detection.
Specifically designed for sports medicine professionals, this device is ideal for the treatment and rehabilitation of sports-related injuries. It delivers precise electrical impulses to targeted areas of the body, promoting pain relief, muscle strengthening, and accelerated recovery.

Professional 12-Channel Low-Frequency Electrical Stimulation Machine LGT-2320BE
The LGT-2320BE is an advanced EMS machine, leveraging the power of low-frequency electrical stimulation therapy to achieve remarkable results in body sculpting.
It not only efficiently contours and tones the body but also actively promotes muscle relaxation, relieving tension and soreness. Moreover, it plays a significant role in enhancing skin elasticity, rejuvenating the skin's appearance and suppleness.
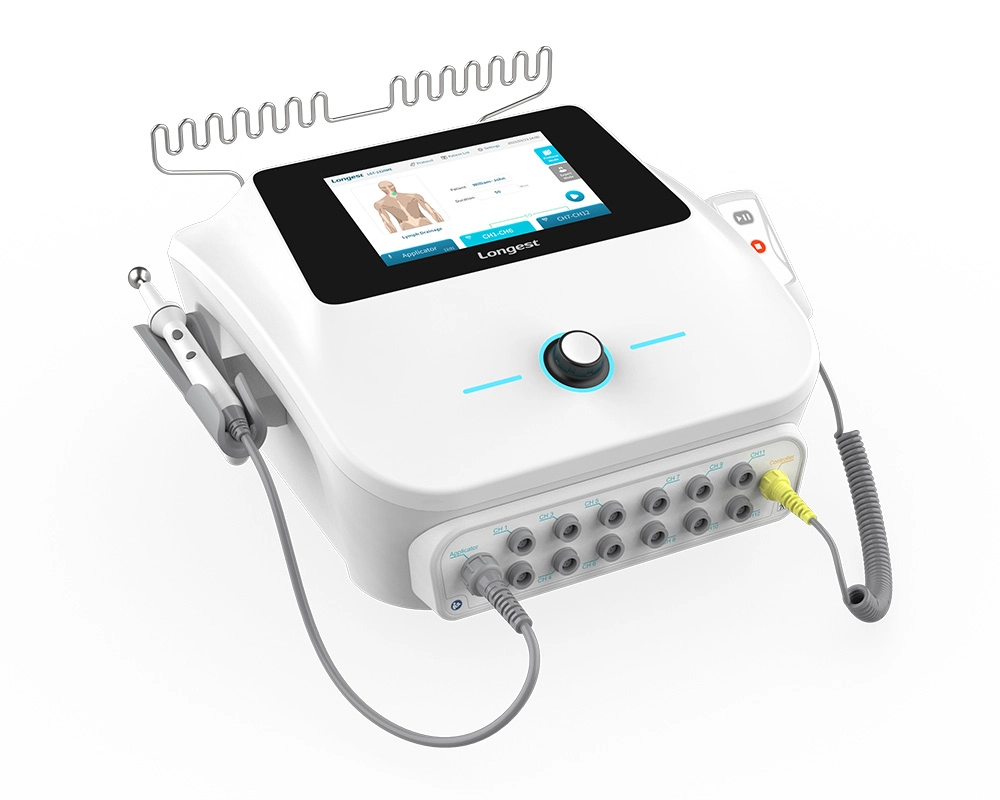
Neuromuscular Electrical Stimulation NMES Machine LGT-2320ME
The LGT-2320ME, a portable electro-stimulation therapy device, is mainly composed of the main unit, a hand switch, a pen electrode controller, and diverse types of electrodes.
It has the capacity to supply three channel groups (CH1-CH6, CH7-CH12, and the Applicator channel) with either TENS or NMES current. Specifically engineered for utilization in hospitals and clinics, this device effectively assists patients in reclaiming lost muscle strength and expedites recovery times, consequently enhancing the overall standard of patient care and bringing about more satisfactory rehabilitation outcomes.

Rehab Bike for Upper and Lower Limbs, Active-Passive Trainer (APT) RehaMoto LGT-5100D
RehaMoto controls the servo motor through the central processing unit and the biomechanical monitoring feedback system. Users can do passive, assisted, active, and constant-speed training by RehaMoto LGT-5100D Config I. The intelligent identification device realizes real - time monitoring of user training status and smooth conversion between different modes. Fully realize the best clinical training effect, and promote the recovery of users' motor function.
© 2025 Longest Medical. All Rights Reserved. Powered by gooeyun.







LongestMedical
LongestGloba
longest
guangzhou_longest
GzLongest