Wholesale dvt medical device manufacturer and supplier
- Wholesale DVT Medical Device Manufacturer and Supplier: What Buyers Need to Know
- Why choose a specialized dvt medical device manufacturer
- Common commercial keywords to guide suppliers and buyers
- Types of DVT Medical Devices and Their Uses
- Intermittent pneumatic compression (IPC) devices
- Compression garments and stockings
- Foot pumps and portable compression systems
- Clinical Evidence and Guidelines for Mechanical DVT Prophylaxis
- Clinical role of mechanical devices
- What buyers should verify about clinical performance
- Regulatory and Quality Considerations for Wholesale Purchases
- Essential regulatory checks
- Quality management and manufacturing standards
- Procurement Strategies for Wholesale DVT Medical Devices
- Assessing total cost of ownership
- Minimum order quantities (MOQs) and lead times
- OEM and private-label options
- Quality Assurance, Testing, and Clinical Support
- Factory testing and inspection
- Training and clinical implementation support
- Why Longest Medical is a Strong Partner for Wholesale DVT Devices
- Company background and relevant expertise
- Commercial capabilities and customization
- Global service and supply chain considerations
- Practical Checklist for Evaluating a Wholesale DVT Medical Device Supplier
- Core procurement checklist
- Conclusion: How to Move Forward with Wholesale DVT Medical Device Purchases
Wholesale DVT Medical Device Manufacturer and Supplier: What Buyers Need to Know
Deep vein thrombosis (DVT) prevention and treatment devices are essential medical products used across hospitals, rehabilitation centers, post-operative care units, and outpatient clinics. For purchasing managers, distributors, and clinicians seeking a reliable wholesale dvt medical device manufacturer and supplier, understanding product types, clinical evidence, regulatory requirements, and supply-chain factors is critical to making cost-effective and clinically sound buying decisions.
Why choose a specialized dvt medical device manufacturer
Partnering with a manufacturer that specializes in compression therapy and related rehabilitation equipment helps ensure devices are designed for clinical performance and durability. A supplier with experience in medical markets understands hospital procurement cycles, can offer OEM and private-label solutions, and supports product training and after-sales service — all important for high-volume purchases and long-term contracts.
Common commercial keywords to guide suppliers and buyers
When searching or listing products, use commercial intent keywords such as wholesale dvt medical device, bulk compression therapy devices, intermittent pneumatic compression (IPC) supplier, sequential compression device (SCD) manufacturer, OEM DVT device, and medical distributor compression therapy to find vendors that support large orders, custom branding, and international shipping.
Types of DVT Medical Devices and Their Uses
Intermittent pneumatic compression (IPC) devices
IPC devices (also called sequential compression devices or SCDs) use inflatable sleeves and a pump unit to apply cyclical pressure to the limbs, promoting venous return. They are widely used in perioperative care, intensive care units, and for patients who are immobilized or have contraindications to pharmacologic prophylaxis. IPC devices are often a primary product line for dvt medical device suppliers because they combine mechanical simplicity with clinical versatility.
Compression garments and stockings
Graduated compression stockings and thigh/ knee-high garments provide constant external pressure and are commonly used both for prevention in ambulatory patients and for long-term management after DVT. They are lightweight, low-cost, and suitable for outpatient prophylaxis and home care programs.
Foot pumps and portable compression systems
Foot pumps and compact portable compression systems deliver intermittent pressure focused on the calf and foot. Their portability makes them popular for outpatient rehabilitation, patients on long-haul travel, and for home-based prophylaxis programs, especially where mobility is limited but full-sized IPC systems are not practical.
Clinical Evidence and Guidelines for Mechanical DVT Prophylaxis
Clinical role of mechanical devices
Mechanical prophylaxis is an established option in DVT prevention strategies. Clinical guidelines — such as those from major clinical societies — recommend mechanical methods when pharmacologic anticoagulation is contraindicated or as an adjunct to anticoagulants in high-risk surgical patients. IPC and SCD devices are particularly valuable in settings where bleeding risk prevents use of drugs.
What buyers should verify about clinical performance
Ask suppliers for clinical references, performance data, and any peer-reviewed studies supporting the device's efficacy. While many clinical trials focus on outcomes in specific surgical or medical populations, credible suppliers will provide documentation of device testing, pressure profiles, and durability testing to demonstrate consistent delivery of therapeutic compression.
Regulatory and Quality Considerations for Wholesale Purchases
Essential regulatory checks
Before placing a wholesale order, verify the manufacturer's regulatory status for your target markets. In many regions, DVT devices are regulated medical devices that require conformity marks such as CE in Europe or clearance/registration with national authorities like FDA in the United States. If you plan to import, ensure the supplier can provide technical documentation, certificates, and declarations of conformity as needed.
Quality management and manufacturing standards
Buyers should confirm that the supplier operates under a robust quality management system (for example, ISO 13485) and follows good manufacturing practices. Request factory audit reports, production process descriptions, and batch traceability procedures. These measures reduce risk of non-conforming products and support hospital procurement compliance.
Procurement Strategies for Wholesale DVT Medical Devices
Assessing total cost of ownership
When evaluating wholesale dvt medical device offers, compare total cost of ownership (TCO) rather than only unit price. TCO includes warranty terms, expected service life, maintenance costs, availability of spare parts (such as pumps and sleeves), and the supplier's logistics support. A slightly higher unit price may be justified by lower long-term maintenance and reliable after-sales service.
Minimum order quantities (MOQs) and lead times
Wholesale suppliers often set MOQs and lead times that reflect production runs and inventory strategies. Discuss MOQs, sample orders, and staggered deliveries with suppliers to align supply with demand, reduce inventory carrying costs, and secure stable pricing for repeat orders. Request typical lead times for standard IPC units, sleeves, and accessories to plan procurement cycles.
OEM and private-label options
Many manufacturers offer OEM/white-label or private-label programs to distributors and large healthcare groups. Verify design customization limits, labeling and packaging requirements, regulatory responsibilities for branded products, and the minimum quantities required for custom production. This is useful for medical distributors looking to build proprietary product lines under their brand.
Quality Assurance, Testing, and Clinical Support
Factory testing and inspection
Robust testing procedures should include pressure calibration and consistency testing, electrical safety for pump units, sleeve seam/burst testing, and lifecycle testing for repeated inflation cycles. Request certificates of inspection for batches and independent third-party test reports when available.
Training and clinical implementation support
Choose a supplier that provides comprehensive clinical training materials, user manuals, and in-service training for nurses and therapists. Proper application, sizing of garments, and pump settings are essential to device effectiveness and patient comfort — an informed clinical team reduces incidents and improves outcomes.
Why Longest Medical is a Strong Partner for Wholesale DVT Devices
Company background and relevant expertise
Founded in 2000, Longest Medical is a global rehabilitation and aesthetic solutions company focused on non-invasive medical technologies. Their product portfolio includes compression therapy systems, electrotherapy, shock wave therapy, cryotherapy, ultrasound therapy, and active-passive trainers — all of which support rehabilitation, postoperative recovery, neurological rehabilitation, and medical aesthetics. This breadth of experience in rehabilitation equipment positions Longest Medical as a credible wholesale dvt medical device manufacturer and supplier.
Commercial capabilities and customization
As a manufacturer serving international markets, Longest Medical typically supports OEM and private-label solutions, bulk order fulfillment, and distributor partnerships. Buyers can negotiate MOQs, request custom packaging and labeling, and access after-sales training and warranty support. For large-volume procurement, such capabilities streamline product rollout across hospital networks and rehabilitation centers.
Global service and supply chain considerations
Longest Medical’s global presence enables international shipping, product support, and spare-part availability. Purchasing managers should confirm specific certifications and market registrations for the destination country and request product documentation and technical files prior to order placement.
Practical Checklist for Evaluating a Wholesale DVT Medical Device Supplier
Core procurement checklist
- Confirm device type suitability (IPC, stockings, foot pump) for your clinical needs.
- Request regulatory documentation and market clearances for your country.
- Verify quality system certifications and factory inspection reports.
- Ask for clinical references or published evaluations when available.
- Compare warranties, spare-part availability, and expected service intervals.
- Clarify MOQs, lead times, and logistics (incoterms, shipping, customs support).
- Negotiate pricing on bulk tiers and explore OEM/private-label options if needed.
- Arrange for sample units and on-site demos for clinical staff acceptance testing.
Conclusion: How to Move Forward with Wholesale DVT Medical Device Purchases
Purchasing dvt medical device products at wholesale requires balancing clinical effectiveness, regulatory compliance, and commercial terms. Focus on suppliers with proven experience in compression therapy, clear regulatory documentation, robust quality assurance, and the ability to support large orders and aftermarket service. Longest Medical — founded in 2000 and offering a broad rehabilitation equipment portfolio — is an example of a manufacturer that can supply compression therapy and related devices to meet institutional needs. Contact potential suppliers early to verify certifications, request samples, and align delivery schedules with your procurement plan.
For distributors and healthcare buyers looking for a wholesale dvt medical device partner: request detailed technical files, ask for clinical training materials, and negotiate TCO-based pricing to ensure the best value for your organization.
Frequently Asked Questions
Q: What is a DVT medical device and when is it used?A: A DVT medical device refers to mechanical products such as intermittent pneumatic compression (IPC) units, sequential compression devices (SCDs), foot pumps, and graduated compression stockings. They are used for prophylaxis and treatment support in hospitalized patients, post-surgical care, immobilized individuals, and outpatient settings where preventing venous thromboembolism is a priority.
Q: Are mechanical DVT devices effective compared to anticoagulants?A: Mechanical devices are a recommended option, particularly when anticoagulants are contraindicated due to bleeding risk. Clinical guidelines support using IPC as an alternative or adjunct to pharmacologic prophylaxis in certain patient populations. Effectiveness depends on correct device selection, sizing, and clinical application.
Q: What regulatory documentation should I request from a manufacturer?A: Ask for declarations of conformity, CE marking documentation if applicable, ISO quality certificates (such as ISO 13485), product technical files, and evidence of registration or clearance in your target market. Also request test reports for pressure performance, electrical safety, and lifecycle testing.
Q: Can I order OEM or private-label DVT devices in bulk?A: Yes. Many manufacturers offer OEM and private-label programs. Confirm minimum order quantities, design customization limits, labeling responsibilities, and any regulatory obligations associated with rebranding medical devices.
Q: How do I ensure clinical staff will accept the devices?A: Arrange product demos and trials, provide clinical training and user manuals, and secure sample units for acceptance testing. Involving nurses and therapists early in evaluation helps ensure correct sizing, application, and settings for optimal patient outcomes.
Q: What are typical warranty and service expectations for wholesale purchases?A: Warranties vary but commonly cover defects in materials and workmanship for a defined period (often 12–24 months). Ensure the supplier provides spare parts availability, technical support channels, and options for extended service agreements for high-volume deployments.
Q: How should I compare suppliers on price?A: Evaluate total cost of ownership: unit price, warranty terms, spare parts and consumable pricing (e.g., sleeves), expected service life, shipping, and after-sales support. Bulk discounts and predictable lead times are also important for budgeting and supply continuity.
Q: How do I verify product quality before placing a large order?A: Request samples, factory audit reports, third-party test certificates, and batch inspection reports. Conduct a pilot deployment in a limited clinical area to verify performance and user acceptance before scaling procurement.
How to Vet China Medical Device Manufacturers for Physio Gear
Active Passive Trainer Cost in Dallas: Complete Buyer’s Guide
Active Passive Trainer Cost in Seattle: Complete Buying Guide
Active Passive Trainer Cost in Spain: Buyer’s Guide for Clinics and Suppliers
Cryotherapy
What are the benefits of cryotherapy?
· Cryotherapy offers several potential benefits, including skin rejuvenation, reduction of inflammation and swelling, targeting cellulite, and potential athletic performance and recovery benefits.
· Cryotherapy can help improve skin tone, reduce the appearance of wrinkles and sagging skin, promote collagen production, and tighten the skin.
· It can also aid in reducing inflammation and swelling, making it beneficial for post - surgical recovery and managing conditions like arthritis.
· Additionally, cryotherapy shows promise in targeting cellulite and aiding in body sculpting efforts. Athletes often turn to cryotherapy for faster recovery, reduced muscle soreness, and improved performance.
LGT-2500S - Beauty
In which circumstances is AWT not suitable?
Pregnancy
Over major blood vessels and nerves
Having pacemakers or other implanted devices
Open wounds
Taking oral anti-coagulants Having a blood clotting disorder
Having received a Steroid injection within 6 weeks
Tumors are present at the treatment site
Having skin infection or abrasion at the
Treatment site
Over lung
LGT-2320ME
Can we use the pen electrode directly over skin?
No, you have to put a wet gauze over the electrode or put some conductive gel over the skin before using it.
LGT-2500S
How to avoid adverse reactions?
① Control intensity reasonably, and strenuous exercise is strictly prohibited within 24 hours after treatment. ② Hot compresses are strictly prohibited within 24 hours after treatment.
Pressotherapy
Is pressotherapy safe?
Pressotherapy is a non - invasive and pain - free treatment. During the process, it feels akin to a massage, leaving you feeling refreshed and revitalized afterwards.

Professional 12-Channel Low-Frequency Electrical Stimulation Machine LGT-2320SP
LGT-2320SP is an advanced electrical stimulation sports training station. It leads to multisite high-efficiency strength gain and realizes bilateral balanced development with motor point detection.
Specifically designed for sports medicine professionals, this device is ideal for the treatment and rehabilitation of sports-related injuries. It delivers precise electrical impulses to targeted areas of the body, promoting pain relief, muscle strengthening, and accelerated recovery.

Professional 12-Channel Low-Frequency Electrical Stimulation Machine LGT-2320BE
The LGT-2320BE is an advanced EMS machine, leveraging the power of low-frequency electrical stimulation therapy to achieve remarkable results in body sculpting.
It not only efficiently contours and tones the body but also actively promotes muscle relaxation, relieving tension and soreness. Moreover, it plays a significant role in enhancing skin elasticity, rejuvenating the skin's appearance and suppleness.
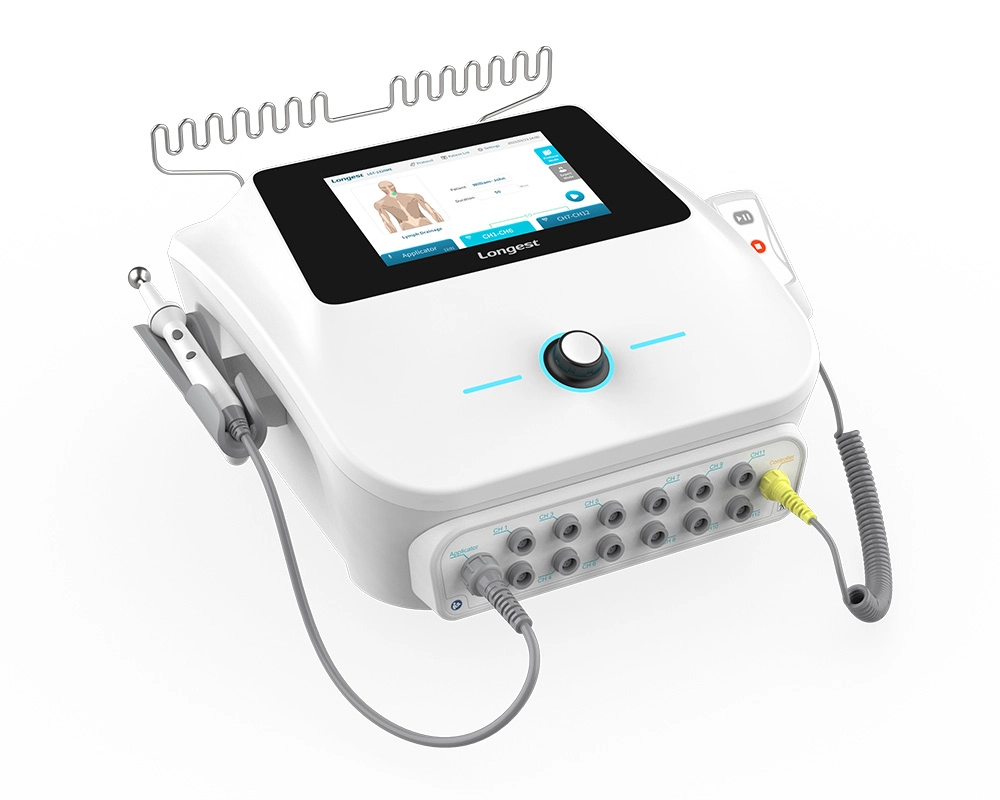
Neuromuscular Electrical Stimulation NMES Machine LGT-2320ME
The LGT-2320ME, a portable electro-stimulation therapy device, is mainly composed of the main unit, a hand switch, a pen electrode controller, and diverse types of electrodes.
It has the capacity to supply three channel groups (CH1-CH6, CH7-CH12, and the Applicator channel) with either TENS or NMES current. Specifically engineered for utilization in hospitals and clinics, this device effectively assists patients in reclaiming lost muscle strength and expedites recovery times, consequently enhancing the overall standard of patient care and bringing about more satisfactory rehabilitation outcomes.

Rehab Bike for Upper and Lower Limbs, Active-Passive Trainer (APT) RehaMoto LGT-5100D
RehaMoto controls the servo motor through the central processing unit and the biomechanical monitoring feedback system. Users can do passive, assisted, active, and constant-speed training by RehaMoto LGT-5100D Config I. The intelligent identification device realizes real - time monitoring of user training status and smooth conversion between different modes. Fully realize the best clinical training effect, and promote the recovery of users' motor function.
© 2025 Longest Medical. All Rights Reserved. Powered by gooeyun.






LongestMedical
LongestGloba
longest
guangzhou_longest
GzLongest