Wholesale sequential compression device for dvt manufacturer and supplier
- Wholesale Sequential Compression Device for DVT: Manufacturer and Supplier Guide
- Introduction: Why buy sequential compression device for DVT in wholesale
- Clinical need and market demand for sequential compression device for DVT
- Types of sequential compression device for DVT manufacturers supply
- Key features buyers should prioritize in a sequential compression device for DVT
- Clinical evidence and guideline support for sequential compression device for DVT
- Regulatory and quality standards for manufacturers of sequential compression device for DVT
- Manufacturing considerations: OEM, ODM and customization options
- Quality control and testing protocols for sequential compression device for DVT
- Supply chain and logistics for bulk sequential compression device for DVT
- Pricing and total cost of ownership considerations
- Choosing the right supplier: evaluation checklist
- Why Longest Medical is a strong partner for wholesale sequential compression device for DVT
- Implementation, training, and clinical integration
- Service, maintenance and warranty for wholesale purchases
- Case applications: where sequential compression device for DVT adds immediate value
- Sustainability and environmental considerations for bulk procurement
- Conclusion: How to proceed when buying wholesale sequential compression device for DVT
Wholesale Sequential Compression Device for DVT: Manufacturer and Supplier Guide
Introduction: Why buy sequential compression device for DVT in wholesale
Hospitals, clinics, and medical distributors increasingly seek to buy wholesale sequential compression device for DVT to meet high-volume demand for venous thromboembolism (VTE) prevention and postoperative care. A sequential compression device for DVT, also called an intermittent pneumatic compression (IPC) system, helps reduce deep vein thrombosis risk by simulating the calf-muscle pump and improving venous return. For procurement teams, choosing a reliable manufacturer and supplier is critical to ensure clinical efficacy, regulatory compliance, and cost-efficiency when purchasing sequential compression device for DVT in bulk.
Clinical need and market demand for sequential compression device for DVT
The global incidence of venous thromboembolism is estimated at roughly 1 to 2 per 1,000 people annually, with hospitalization and surgery significantly increasing risk. Clinical guidelines recommend mechanical prophylaxis, such as a sequential compression device for DVT, particularly when anticoagulant therapy is contraindicated or as adjunctive therapy after major orthopedic and trauma surgery. This clinical imperative drives steady demand from hospitals, rehabilitation centers, and outpatient surgical clinics looking to acquire sequential compression device for DVT at wholesale prices.
Types of sequential compression device for DVT manufacturers supply
Manufacturers produce several configurations of sequential compression device for DVT to suit different clinical settings. Common types include calf-length sleeves, thigh-length sleeves, and full-leg systems with multi-chamber sequential inflation. Some suppliers offer portable single-channel compressors for home use and compact multi-channel units for intensive hospital use. When sourcing a wholesale sequential compression device for DVT, buyers should evaluate sleeve materials (disposable vs reusable), inflation pressures, cycle patterns, and number of channels to match patient population and use case.
Key features buyers should prioritize in a sequential compression device for DVT
When selecting a wholesale sequential compression device for DVT, prioritize clinically proven features: programmable sequential compression cycles, adjustable inflation pressure, multi-chamber sleeves for distal-to-proximal compression, easy-to-clean surfaces, alarm systems, and portability options. Commercial buyers should also look for energy-efficient compressors and compatibility with disposable sleeves to reduce cross-contamination risks. These product features directly impact clinical outcomes and the total cost of ownership for sequential compression device for DVT fleets.
Clinical evidence and guideline support for sequential compression device for DVT
Major clinical guidelines support mechanical prophylaxis for DVT in patients at increased bleeding risk or when pharmacologic prophylaxis is unsuitable. For example, guideline bodies recommend using IPC devices in high-risk surgical patients and in combination with anticoagulants when indicated. Clinical trials have demonstrated that appropriately used sequential compression device for DVT reduce venous stasis and lower DVT incidence in hospitalized and postoperative populations, supporting procurement decisions to buy wholesale sequential compression device for DVT for institutional programs.
Regulatory and quality standards for manufacturers of sequential compression device for DVT
Buyers should ensure manufacturers of sequential compression device for DVT meet international quality and safety standards. Look for ISO 13485 certification for medical device quality management, CE marking for sale in the European Economic Area, and FDA 510(k) clearance for markets in the United States where applicable. Compliance with IEC 60601-series standards for electrical safety and electromagnetic compatibility is also important for powered compressors. Choosing certified manufacturers reduces regulatory risk when buying wholesale sequential compression device for DVT.
Manufacturing considerations: OEM, ODM and customization options
Many suppliers offer OEM and ODM services, allowing buyers to brand sequential compression device for DVT products or request design variations for specific clinical workflows. Wholesale buyers should discuss minimum order quantities (MOQs), lead times, and prototyping capabilities with manufacturers. Customizable options for sequential compression device for DVT often include bespoke pressure profiles, screen and control layouts, accessory compatibility, and packaging for private-label distribution.
Quality control and testing protocols for sequential compression device for DVT
Reliable manufacturers implement rigorous testing protocols including cycle durability testing, pressure accuracy verification, sleeve leak testing, biocompatibility for patient-contact materials, and electrical safety inspections. When sourcing a wholesale sequential compression device for DVT, request factory test reports, batch traceability documentation, and sample test results. Robust quality control ensures each unit functions properly in clinical environments and reduces product failure risks after deployment.
Supply chain and logistics for bulk sequential compression device for DVT
Procurement teams buying wholesale sequential compression device for DVT should evaluate supplier capabilities for inventory management, shipping, and after-sales support. Important logistics factors include production lead times, FOB/CIF pricing, warehousing options, and ability to handle phased deliveries for large hospital networks. Reliable suppliers provide spare parts distribution, training materials, and local service partnerships to support clinical continuity when deploying sequential compression device for DVT at scale.
Pricing and total cost of ownership considerations
Wholesale pricing for sequential compression device for DVT depends on unit configuration, features, certification status, and order volume. Buyers should look beyond unit price and calculate total cost of ownership: maintenance, consumables (disposable sleeves), warranty, accessories, training, and expected device lifespan. Bulk purchases typically yield volume discounts and lower per-unit shipping costs, making it cost-effective to buy wholesale sequential compression device for DVT when planning multi-site deployments.
Choosing the right supplier: evaluation checklist
When selecting a manufacturer or supplier to buy sequential compression device for DVT in wholesale, use a checklist: regulatory certifications (ISO 13485, CE, FDA), clinical evidence and validation, production capacity and lead times, OEM/ODM flexibility, quality control documentation, warranty and after-sales service, references from hospitals or distributors, and commercial terms for bulk orders. A thorough supplier evaluation reduces procurement risk and ensures consistent supply of sequential compression device for DVT.
Why Longest Medical is a strong partner for wholesale sequential compression device for DVT
Founded in 2000, Longest Medical is a global rehabilitation and aesthetic solutions company focusing on non-invasive medical technologies. With product lines spanning compression therapy, shock wave therapy, electrotherapy, cryotherapy, ultrasound therapy, and active-passive trainers, Longest Medical delivers comprehensive equipment solutions for physical therapy, neurological rehabilitation, postoperative recovery, veterinary care, and medical aesthetics. As a manufacturer and supplier, Longest Medical offers certified devices, OEM/ODM services, and experience handling bulk orders of sequential compression device for DVT for institutional clients.
Implementation, training, and clinical integration
To maximize clinical benefit when deploying sequential compression device for DVT, suppliers should provide user training, clinical protocols, and integration guidance for nursing and physiotherapy teams. Training topics typically include device setup, patient selection, contraindications, pressure settings, sleeve fitting, cleaning and disinfection, and troubleshooting. Purchasing sequential compression device for DVT in bulk should include plans for staff education to ensure safe and consistent use across sites.
Service, maintenance and warranty for wholesale purchases
Long-term value from sequential compression device for DVT depends on accessible maintenance and warranty coverage. When buying wholesale, negotiate service-level agreements (SLAs), spare parts kits, and extended warranties. Routine maintenance often involves cleaning protocols, inspection for sleeve integrity, compressor filter changes, and functional checks. A supplier with regional service partners reduces downtime for critical wound and VTE prevention programs.
Case applications: where sequential compression device for DVT adds immediate value
Sequential compression device for DVT are widely used in orthopedic surgery recovery, trauma units, stroke rehabilitation, obstetrics for postpartum VTE prevention, and outpatient recovery programs. Institutions buying wholesale sequential compression device for DVT can deploy devices across perioperative units, inpatient wards, and outpatient clinics to standardize prophylaxis protocols and reduce variability in DVT prevention practices.
Sustainability and environmental considerations for bulk procurement
Buyers are increasingly evaluating environmental impact when sourcing sequential compression device for DVT. Consider suppliers that offer recyclable packaging, reusable sleeves with validated reprocessing protocols, and energy-efficient compressors. Sustainable procurement choices can help hospitals meet environmental targets while still acquiring effective sequential compression device for DVT solutions in wholesale quantities.
Conclusion: How to proceed when buying wholesale sequential compression device for DVT
Purchasing sequential compression device for DVT in wholesale offers cost and operational advantages for healthcare networks, but success depends on selecting a certified manufacturer and supplier with robust quality systems, clinical validation, and after-sales support. Assess product features, regulatory status, OEM/ODM options, total cost of ownership, and logistics readiness. Longest Medical, with two decades of experience and comprehensive rehabilitation product lines, is positioned to supply high-quality sequential compression device for DVT at scale and support clinical programs from procurement through implementation.
Frequently Asked Questions
What is a sequential compression device for DVT and how does it work?A sequential compression device for DVT is an intermittent pneumatic compression system that inflates multi-chamber sleeves around the limb in sequence to mimic the natural calf-muscle pump, improving venous return and lowering the risk of clot formation.
When should a hospital buy sequential compression device for DVT in bulk?Hospitals should consider bulk procurement when implementing system-wide VTE prophylaxis protocols, expanding perioperative services, or equipping multiple wards and outpatient clinics to ensure device availability and reduce unit cost.
Are sequential compression device for DVT effective compared to anticoagulants?IPC devices are recommended as mechanical prophylaxis, particularly when anticoagulants are contraindicated. They are often used alongside pharmacologic prophylaxis in high-risk patients; choice depends on patient bleeding risk and clinical guidelines.
What certifications should I ask for when choosing a supplier of sequential compression device for DVT?Request ISO 13485, CE marking for EU markets, and FDA 510(k) clearance where applicable. Also verify compliance with IEC 60601 electrical safety standards and ask for factory quality control documentation.
Can sequential compression device for DVT be customized for private label distribution?Yes, many manufacturers offer OEM/ODM services for private labeling, custom packaging, and tailored features. Discuss MOQs, lead times, and prototyping with the supplier early in the procurement process.
How do I estimate the total cost of ownership for wholesale sequential compression device for DVT?Include unit price, shipping, consumables (disposable sleeves), maintenance, training, warranty, expected device lifespan, and potential downtime in your calculations to determine the true cost per patient or per use.
What support should a supplier provide after a bulk purchase?Suppliers should offer commissioning support, user training, technical documentation, spare parts availability, remote or onsite service options, and clear warranty and SLA terms to ensure uninterrupted clinical use of sequential compression device for DVT.
Active Passive Trainer Cost in Washington: Complete Buyer’s Guide
Wholesale Lymphatic drainage massage machine manufacturer and supplier
Wholesale active passive trainer manufacturer and supplier
Tips for buy best cardiac rehab equipment
LGT - 2510B
What are the benefits of shockwave treatment?
The benefits of shockwave treatment include alleviating chronic pain, stimulating the body's natural healing response, promoting tissue regeneration, increasing blood circulation, and restoring mobility and functionality.
LGT-2200SP
Can I use LGT - 2200SP at home?
Yes, the LGT-2200SP is specially designed for individuals to receive effective lymphatic drainage air compression therapy and enjoy personal relaxation in the comfort of their own homes. It is intended to improve poor circulation, enhance muscle flexibility, and provide the peace of mind that comes with self-care.
Kinesiotherapy
Can you provide any specific certifications or credentials that the rehab bike meets to ensure quality and safety standards?
Yes. We can provide you with the ISO 13485 certificate, CE certificate, TGA certificate, NRTL certificate, and Health Canada registration certificate.
LGT-2900EUV
How does the device manage patient data and ensure security?
Privacy protection: A mandatory account login system restricts access to patient databases, complying with medical data security standards.
Data tracking: Records patient profiles, treatment history (protocols, parameters, session frequency), and outcomes for easy efficacy monitoring and protocol adjustment.
Software updates: USB-enabled system upgrades provide new protocols and features, keeping the device compatible with evolving clinical practices.
2510A-beauty
Is there any pain or discomfort during the treatment process of acoustic wave therapy?
Usually, the pain or discomfort is mild. During treatment, when the energy is high or it targets sensitive areas, there may be a brief stinging or warm feeling. It's usually bearable, and beauticians or doctors can adjust the parameters based on patient feedback.

Professional 12-Channel Low-Frequency Electrical Stimulation Machine LGT-2320SP
LGT-2320SP is an advanced electrical stimulation sports training station. It leads to multisite high-efficiency strength gain and realizes bilateral balanced development with motor point detection.
Specifically designed for sports medicine professionals, this device is ideal for the treatment and rehabilitation of sports-related injuries. It delivers precise electrical impulses to targeted areas of the body, promoting pain relief, muscle strengthening, and accelerated recovery.

Professional 12-Channel Low-Frequency Electrical Stimulation Machine LGT-2320BE
The LGT-2320BE is an advanced EMS machine, leveraging the power of low-frequency electrical stimulation therapy to achieve remarkable results in body sculpting.
It not only efficiently contours and tones the body but also actively promotes muscle relaxation, relieving tension and soreness. Moreover, it plays a significant role in enhancing skin elasticity, rejuvenating the skin's appearance and suppleness.
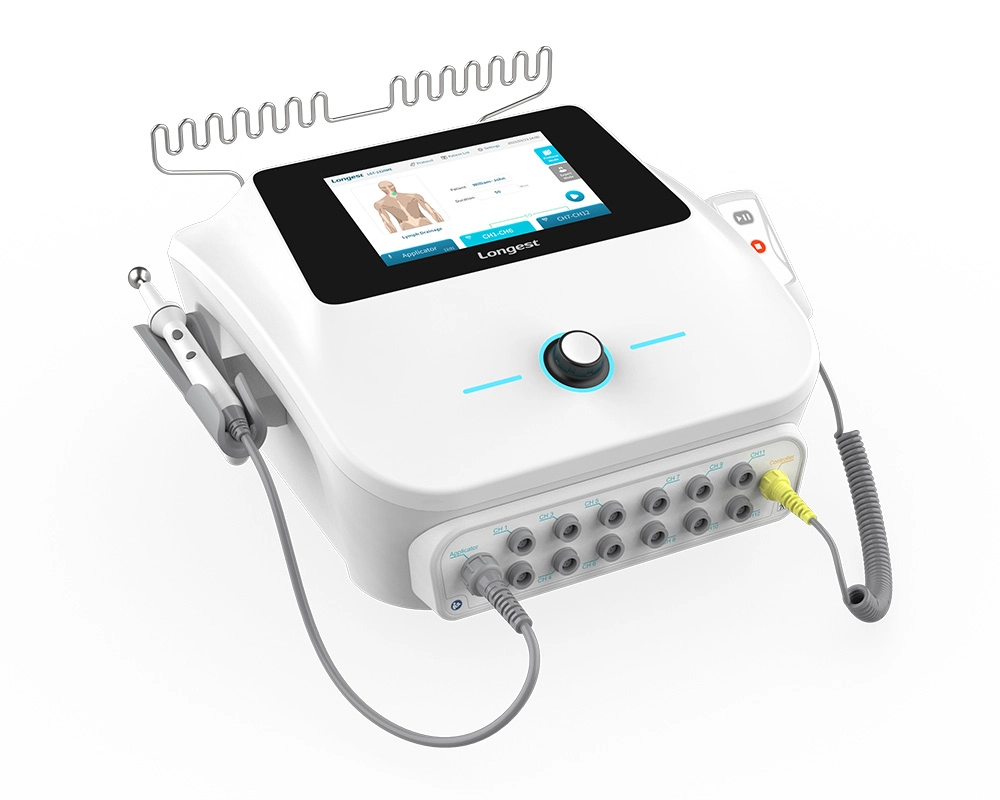
Neuromuscular Electrical Stimulation NMES Machine LGT-2320ME
The LGT-2320ME, a portable electro-stimulation therapy device, is mainly composed of the main unit, a hand switch, a pen electrode controller, and diverse types of electrodes.
It has the capacity to supply three channel groups (CH1-CH6, CH7-CH12, and the Applicator channel) with either TENS or NMES current. Specifically engineered for utilization in hospitals and clinics, this device effectively assists patients in reclaiming lost muscle strength and expedites recovery times, consequently enhancing the overall standard of patient care and bringing about more satisfactory rehabilitation outcomes.

Rehab Bike for Upper and Lower Limbs, Active-Passive Trainer (APT) RehaMoto LGT-5100D
RehaMoto controls the servo motor through the central processing unit and the biomechanical monitoring feedback system. Users can do passive, assisted, active, and constant-speed training by RehaMoto LGT-5100D Config I. The intelligent identification device realizes real - time monitoring of user training status and smooth conversion between different modes. Fully realize the best clinical training effect, and promote the recovery of users' motor function.
© 2025 Longest Medical. All Rights Reserved. Powered by gooeyun.






LongestMedical
LongestGloba
longest
guangzhou_longest
GzLongest