Regulatory Checklist: FDA & CE Compliance for Shockwave Devices
- Regulatory Roadmap for Shockwave Devices: FDA & CE Compliance Essentials
- Device classification and intended use for shockwave therapy machine
- Technical documentation checklist for a shockwave therapy machine (FDA & CE)
- 510(k) premarket route essentials for a shockwave therapy machine
- MDR conformity assessment for shockwave therapy machine (CE marking)
- Comparative checklist: FDA 510(k) vs EU CE (MDR) for shockwave therapy machine
- Clinical evidence strategy for a shockwave therapy machine
- Key testing standards and laboratory requirements for shockwave therapy machine
- Quality Management Systems and manufacturing controls for shockwave therapy machine
- Common pitfalls and mitigations when registering a shockwave therapy machine
- Pricing and timeline expectations for regulatory approval of a shockwave therapy machine
- Integrating vendor selection: choosing a compliant shockwave therapy machine supplier
- Company profile — Longest Medical and shockwave therapy machine solutions
- Practical next steps checklist before submission for your shockwave therapy machine
- FAQ — Common questions about FDA & CE compliance for shockwave therapy machine
- 1. Do all shockwave therapy machines need clinical trials for CE marking?
- 2. Is a 510(k) required for every shockwave therapy machine in the U.S.?
- 3. What are the essential tests for energy output and safety?
- 4. How should I document software in a shockwave therapy machine?
- 5. How long does CE MDR approval typically take for a shockwave therapy machine?
- Contact & product inquiry — accelerate your shockwave therapy machine compliance
- References
Regulatory Roadmap for Shockwave Devices: FDA & CE Compliance Essentials
Device classification and intended use for shockwave therapy machine
Before preparing submissions, confirm the device’s intended use and technological characteristics. Shockwave therapy machine products (extracorporeal and focused shock wave devices) intended for musculoskeletal, wound healing, or aesthetic indications are typically regulated as medical devices. In the U.S., many shockwave therapy systems have historically been cleared under FDA 510(k) as Class II devices with predicate devices; some high-energy lithotripsy systems differ in classification. In the EU, under the Medical Device Regulation (EU) 2017/745 (MDR), these devices are commonly classified as Class IIa or IIb depending on invasiveness, duration of contact and clinical claims. Accurate classification drives the conformity assessment route, clinical data needs and testing scope.
Technical documentation checklist for a shockwave therapy machine (FDA & CE)
Technical documentation (also called design dossier or technical file) is the backbone of any submission. Below are essential elements to compile:
- Device description and intended purpose, device variants and accessories.
- Design drawings, bill of materials, software architecture and lock-down (if applicable).
- Risk management file per ISO 14971 — hazards, risk analysis, mitigation and residual risk acceptability.
- Biocompatibility evaluation (ISO 10993) for patient-contact components.
- Electromagnetic compatibility and electrical safety testing per IEC 60601 series.
- Performance testing: energy output characterization, device accuracy, reproducibility, transducer performance and dosimetry.
- Sterilization & shelf-life (if applicable), packaging validation.
- Software lifecycle documentation per IEC 62304, and cybersecurity considerations.
- Manufacturing process validation and supplier control records.
- Clinical evaluation report (CER) for EU MDR, and clinical evidence summary for FDA when required.
- Labeling, IFU (instructions for use), promotional material and UDI plan (EU & US).
510(k) premarket route essentials for a shockwave therapy machine
Most manufacturers targeting the U.S. market will prepare a 510(k) unless a de novo or PMA is required. Key points:
- Identify appropriate predicate device(s) in the FDA device classification database and product code.
- Demonstrate substantial equivalence in intended use and technological characteristics, or provide data to address differences.
- Include bench testing, electrical safety (IEC 60601), EMC, software verification and validation, and relevant performance testing.
- Provide clinical data if non-clinical testing is insufficient to demonstrate safety/effectiveness for claimed indications.
- Quality system compliance with 21 CFR Part 820 (QMS) is expected during inspection; state your QMS status and procedures.
MDR conformity assessment for shockwave therapy machine (CE marking)
Under EU MDR the conformity assessment is more stringent than the former MDD. For a shockwave therapy machine:
- Determine device classification per MDR Annex VIII: many shockwave therapy devices fall into Class IIa or IIb depending on claims and duration/intensity of patient contact.
- Engage a Notified Body early; for Class IIa/IIb devices, a conformity assessment with a Notified Body is required.
- Prepare a robust Clinical Evaluation Report (CER) conforming to MEDDEV/MDCG guidance which assesses clinical literature, equivalence, and any clinical investigations.
- Implement UDI and post-market surveillance (PMS) plans, including post-market clinical follow-up (PMCF) proportionate to risk.
- Maintain a Technical Documentation file and a Declaration of Conformity (DoC) once conformity is established.
Comparative checklist: FDA 510(k) vs EU CE (MDR) for shockwave therapy machine
The table below highlights the principal differences and similar requirements for the two major markets.
| Topic | FDA (510(k)) | EU (MDR) |
|---|---|---|
| Device Classification | Typically Class II (predicate-based); check product code. | Often Class IIa or IIb per Annex VIII; classification rule dependent. |
| Conformity Route | 510(k) premarket notification; de novo or PMA if no predicate. | Notified Body conformity assessment; full technical documentation and CER required. |
| Clinical Evidence | Bench data often sufficient; clinical data required if substantial equivalence can’t be shown. | Comprehensive CER required; PMCF often required post-market. |
| QMS | 21 CFR Part 820 (FDA QSR). | ISO 13485 expectation and Notified Body audit. |
| Standards | IEC 60601, IEC 62304, ISO 14971 typical references. | Same harmonized standards (if harmonized under MDR) + ISO 13485. |
| Labeling & UDI | UDI required, labeling guidance per FDA. | UDI required, MDR labeling and IFU rules stricter. |
| Timeline | Typical 90 days review for 510(k) after submission completeness. | Variable: Notified Body review may take months; depends on NB workload and completeness. |
Clinical evidence strategy for a shockwave therapy machine
Clinical evidence expectations have increased globally. For CE under MDR, the CER must systematically assess available literature, equivalence justification (if used), and any clinical investigations. For FDA, a well-structured clinical summary or clinical study may be necessary if bench testing cannot address safety/effectiveness questions.
Practical approach:
- Conduct a systematic literature review focusing on the device type (focused vs radial shockwave), energy levels, indication (e.g., plantar fasciitis, tendinopathy), and outcomes.
- Generate high-quality bench testing and performance characterization (dose-response, energy flux density measurements).
- Plan clinical investigations only when gaps exist — design per GCP, with endpoints aligned to labeling claims.
- Document PMCF/PMM to capture real-world evidence post-market and support ongoing safety and performance under MDR.
Key testing standards and laboratory requirements for shockwave therapy machine
Laboratory testing typically includes:
- Electrical safety and EMC: IEC 60601-1 and IEC 60601-1-2.
- Acoustic and energy measurements specific to shockwave devices (specialized metrology labs).
- Biocompatibility: ISO 10993 series for patient-contact materials.
- Software lifecycle and cyber-security testing: IEC 62304 and ISO/IEC 27001 considerations where applicable.
Choose notified labs or FDA-accepted testing facilities and retain traceable test reports and calibration certificates.
Quality Management Systems and manufacturing controls for shockwave therapy machine
A mature QMS minimizes regulatory risk. Implement ISO 13485-aligned QMS and document supplier controls, production validation, incoming inspection, traceability, complaint handling, CAPA and management review. For US market readiness, prepare for FDA QSR inspections and demonstrate design controls, verification/validation records and device history records.
Common pitfalls and mitigations when registering a shockwave therapy machine
Manufacturers frequently encounter recurring issues:
- Poorly defined intended use or over-broad claims — ensure claims are supported by evidence.
- Insufficient risk analysis or missing residual risk justification — maintain a current ISO 14971 file.
- Inadequate performance characterization (energy output reproducibility) — invest in robust metrology.
- Underestimating Notified Body or FDA review timelines — build contingency time into launch plans.
- Neglecting post-market surveillance or device vigilance systems — implement early to satisfy MDR/PMS obligations.
Pricing and timeline expectations for regulatory approval of a shockwave therapy machine
Costs and timelines vary by device complexity and evidence gaps. Typical ranges (indicative):
| Stage | Estimated Time | Indicative Cost Range (USD/EUR) |
|---|---|---|
| Bench testing & lab reports | 1–6 months | 10k–80k |
| Clinical study (if needed) | 6–36 months | 50k–1M+ |
| 510(k) prep & submission | 3–6 months | 15k–100k (consulting, testing) |
| CE MDR NB assessment | 3–12 months | 20k–200k (NB fees, audits) |
Note: Costs are highly variable depending on laboratory fees, clinical needs and regulatory consultancy. Build a margin for iterations.
Integrating vendor selection: choosing a compliant shockwave therapy machine supplier
When procuring devices, evaluate suppliers on regulatory documentation readiness (TD, test reports), QMS certification (ISO 13485), history of market clearances (510(k), CE Mark), and post-sales support (training, service, spare parts). For cross-border supply, check import/export compliance and local registration requirements.
Company profile — Longest Medical and shockwave therapy machine solutions
Founded in 2000, Longest Medical is a leading global rehabilitation and aesthetic solutions company, focusing on non-invasive medical solutions. Its products include shock wave therapy, compression therapy, electrotherapy, electrostatic oscillation therapy, cryotherapy, ultrasound therapy, and active-passive trainers. These product lines provide comprehensive equipment solutions for physical therapy, neurological rehabilitation, postoperative recovery, veterinary diagnosis and treatment, medical aesthetics, and other fields.
Longest’s shockwave therapy machine and focused shockwave therapy machine offerings are engineered for reproducible energy delivery, robust metrology and user-focused interfaces. Complementary devices include electrical muscle stimulation machine, air relax compression, active passive trainer, compression therapy machine, DVT medical device, lymphatic massage device and Pressotherapy machine. Key competitive advantages:
- Broad product portfolio allowing bundled solutions across rehabilitation and aesthetics.
- Two decades of industrial experience (since 2000) with established international distribution and service networks.
- Focus on non-invasive technologies with proven clinical use cases in physiotherapy and postoperative recovery.
- Engineering emphasis on reproducible output and compliance to international standards to ease regulatory submissions.
- Integrated after-sales support including training, spare parts and maintenance programs tailored for clinics and hospitals.
Practical next steps checklist before submission for your shockwave therapy machine
Use this concise action list as you prepare for market entry:
- Finalize intended use and device labeling (clear, supportable claims).
- Complete risk management (ISO 14971) and identify required tests/standards.
- Commission accredited labs for IEC 60601, EMC, biocompatibility and acoustic energy testing.
- Prepare clinical evaluation (literature review) and plan clinical studies only if gaps remain.
- Implement or align QMS with ISO 13485; prepare design history files and manufacturing controls.
- Engage with a Notified Body (EU) or assemble a 510(k) dossier and identify predicates (US).
- Plan post-market surveillance, vigilance procedures and UDI implementation.
FAQ — Common questions about FDA & CE compliance for shockwave therapy machine
1. Do all shockwave therapy machines need clinical trials for CE marking?
Not always. For CE under MDR, a thorough clinical evaluation report (CER) is mandatory. If sufficient clinical literature and equivalence data exist to demonstrate safety and performance for the intended use, new clinical investigations may be avoidable. However, manufacturers should be prepared to perform PMCF post-market. (See MDCG and MDR guidance.)
2. Is a 510(k) required for every shockwave therapy machine in the U.S.?
Most shockwave therapy machines that are marketed for therapeutic uses have been cleared via 510(k) as Class II devices using predicates. If no predicate exists or the device presents a new type of risk, a de novo or PMA may be required. Always check the FDA classification database and consult regulatory counsel early.
3. What are the essential tests for energy output and safety?
Essential tests include acoustic/energy output characterization (e.g., energy flux density), reproducibility, transducer integrity, IEC 60601 electrical safety, EMC, and biocompatibility for patient-contact parts (ISO 10993). Use accredited labs with appropriate metrology capability.
4. How should I document software in a shockwave therapy machine?
Document software per IEC 62304: software classification, requirements, architecture, verification/validation, configuration management and cybersecurity risk analysis (ISO 14971 + IEC TR 80002-1 guidance).
5. How long does CE MDR approval typically take for a shockwave therapy machine?
Timeline varies: Notified Body review for Class IIa/IIb devices can take several months to over a year depending on dossier completeness, clinical evidence robustness and NB workload. Early engagement with a chosen Notified Body is recommended.
Contact & product inquiry — accelerate your shockwave therapy machine compliance
If you need regulatory consulting, testing coordination or a compliant device supplier, contact Longest Medical for product specifications, regulatory documentation packages, and technical support. Explore their shockwave therapy machine, focused shockwave therapy machine and complementary rehabilitation solutions to streamline procurement and market entry.
References
- U.S. Food & Drug Administration (FDA) — Device Advice: 510(k) Premarket Notification. https://www.fda.gov/medical-devices/premarket-submissions/premarket-notification-510k (accessed 2025-12-10)
- European Commission — Medical Devices Regulation (MDR) (EU) 2017/745. https://eur-lex.europa.eu/eli/reg/2017/745/oj (accessed 2025-12-10)
- ISO — ISO 13485 Quality Management Systems — Medical devices. https://www.iso.org/iso-13485-medical-devices. (accessed 2025-12-10)
- ISO — ISO 14971 Medical devices — Application of risk management. https://www.iso.org/standard/72704. (accessed 2025-12-10)
- IEC — IEC 60601 series for medical electrical equipment. https://www.iec.ch/medical-technology (accessed 2025-12-10)
- MEDDEV & MDCG guidance on clinical evaluation and clinical investigations (European Commission). Example: MDCG 2020-13 — Guidance on clinical evaluation. https://ec.europa.eu/docsroom/documents/42802 (accessed 2025-12-10)
- Wang CJ. Extracorporeal shockwave therapy in musculoskeletal disorders. J Orthop Surg Res. 2012;7:11. https://pubmed.ncbi.nlm.nih.gov/22642636/ (accessed 2025-12-10)
Wholesale radial shockwave therapy machine manufacturer and supplier
How Electrotherapy Devices Work: A Simple Guide
What is a Compression Therapy Machine? A Complete Guide by Longest Medical
Wholesale Lymphatic drainage massage machine manufacturer and supplier
Company
What is the establishment time and development history of the company?
Guangzhou Longest Medical Technology Co., Ltd. was established in 2000. Over the past 25 years, with research and development as the core, it has gradually built a mature operation system integrating research and development, design, manufacturing, and marketing. Starting from China and expanding globally, its products have been exported to more than 80 countries.
LGT-2320SP
Does your product come with a warranty?
Yes, we're committed to delivering quality products and excellent after-sales service to enhance your overall experience.
2520GP
Does your product come with a warranty?
Yes, we're committed to delivering quality products and excellent after-sales service to enhance your overall experience.
2410S-Mechine
Can the trolley and host be separated?
No, the trolley and the host are integrated and cannot be separated.
Cryotherapy
Can anyone undergo cryotherapy?
Cryotherapy isn't suitable for everyone. People with conditions like pregnancy, severe hypertension, heart problems, certain skin issues, or cold - related allergies should avoid it. Always share your medical history with a cryotherapy professional and consult a healthcare provider if you have concerns.
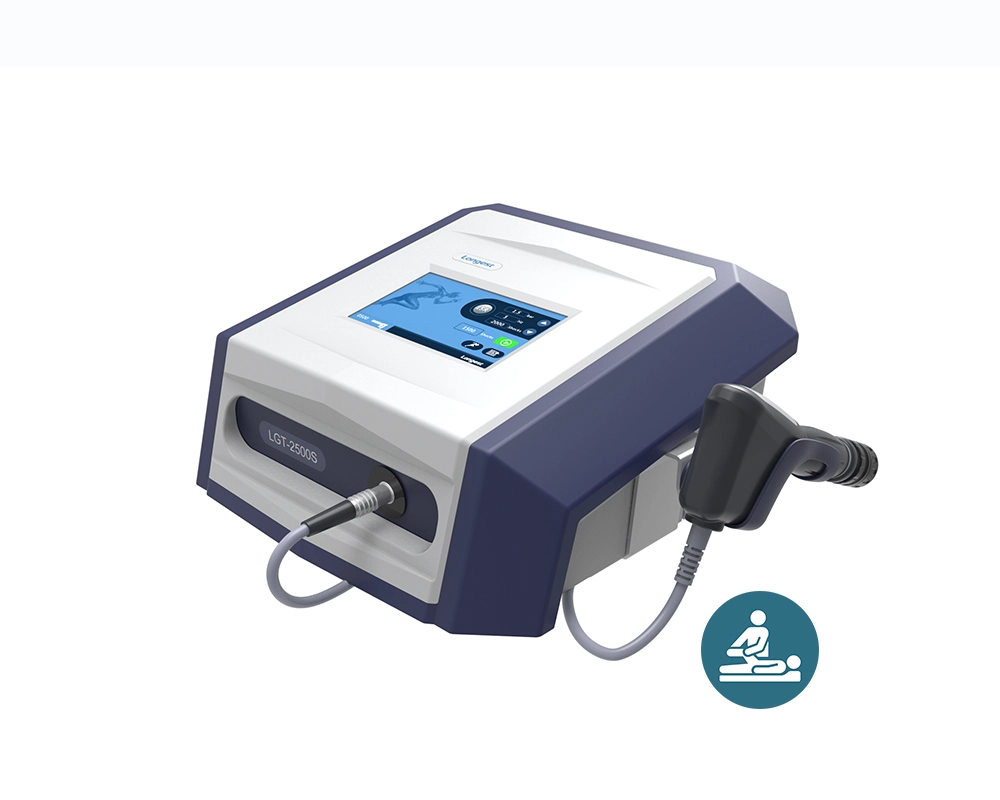
Electrical Extracorporeal Shock Wave Therapy Device PowerShocker LGT-2500S
The LGT-2500S is a portable, tabletop extracorporeal shock wave therapy (ESWT) device. It effectively stimulates the healing of troublesome tissue, alleviates pain, and non-invasively supports the recovery of the patient's tissue. It is a completely natural and drug-free option for various conditions in physiotherapy, veterinary medicine, and aesthetic medicine.

Electromagnetic Stimulator for Musculoskeletal Rehabilitation LGT-2660AP/BP
The LGT-2660 Series Electromagnetic Stimulator for Musculoskeletal Pain is an advanced rehabilitation device engineered to address a wide range of musculoskeletal pain conditions and associated dysfunctions. Operating on the principle of electromagnetic induction, it generates induced currents to regulate neuromuscular function while delivering deeply penetrating energy—this energy transcends superficial tissues to act directly on muscles, joints, and bones. For both acute and chronic musculoskeletal pain, it provides a safe, efficient, and medication-free new solution.

Professional 12-Channel Low-Frequency Electrical Stimulation Machine LGT-2320SP
LGT-2320SP is an advanced electrical stimulation sports training station. It leads to multisite high-efficiency strength gain and realizes bilateral balanced development with motor point detection.
Specifically designed for sports medicine professionals, this device is ideal for the treatment and rehabilitation of sports-related injuries. It delivers precise electrical impulses to targeted areas of the body, promoting pain relief, muscle strengthening, and accelerated recovery.

Electromagnetic Stimulator for Pelvic floor rehabilitation LGT-2640AP
The LGT-2640AP Pelvic Floor Magnetic Stimulator is a precision rehabilitation device designed for targeted pelvic floor therapy. Featuring 24 visualized preset protocols (paired with anatomical diagrams), customizable treatment plans, and a pre-adaptation function, it delivers non-invasive, painless stimulation to support postpartum recovery, male pelvic floor rehabilitation, and geriatric pelvic health.
© 2025 Longest Medical. All Rights Reserved. Powered by gooeyun.






LongestMedical
LongestGloba
longest
guangzhou_longest
GzLongest