Which shockwave therapy devices are FDA-cleared?
- What Are Shockwave Therapy Devices?
- Which Shockwave Therapy Devices Are FDA-Cleared?
- What Are the Regulatory Requirements for Shockwave Therapy Devices?
- What Are the Market Considerations for Procuring Shockwave Therapy Devices?
- How Does Longest Medical Support Medical Device Procurement?
- Conclusion
- References
What Are Shockwave Therapy Devices?
Shockwave therapy devices utilize acoustic waves to promote healing in various musculoskeletal conditions. These devices are categorized into two main types:
- Focused Shockwave Therapy (ESWT): Delivers concentrated energy to deeper tissues for precision treatment.
- Radial Shockwave Therapy (EPAT): Disperses energy over a broader, more superficial area.
The choice between these devices depends on the specific clinical indications and treatment goals. ((https://www.longestmedical.com/b2b-buyers-guide-shockwave-therapy-machine.html?utm_source=openai))
Which Shockwave Therapy Devices Are FDA-Cleared?
Several shockwave therapy devices have received FDA clearance for specific medical applications:
SoftWave OrthoGold 100: Cleared for the treatment of superficial, partial-thickness, second-degree burns in adults and diabetic foot ulcers.
CuraMedix DuoLith SD1 TTOP Ultra: Approved for the treatment of chronic proximal plantar fasciitis.
Zynex TensWave: Cleared for pain management and rehabilitation through transcutaneous electrical nerve stimulation (TENS).
InMode Evolve: An innovative hands-free platform that remodels skin, targets adipose tissue, and tones muscles.
Storz Medical Devices: A range of radial pressure wave (EPAT) and focused shockwave (ESWT) devices, including the DUOLITH® SD1 (FSW + RSW) and MASTERPULS® ULTRA +.
What Are the Regulatory Requirements for Shockwave Therapy Devices?
To obtain FDA clearance, manufacturers must submit a 510(k) premarket notification demonstrating that their device is substantially equivalent to a legally marketed device. The submission should include:
- Device description and predicate device identification.
- Bench testing results: acoustic output, energy flux density (EFD), focal mapping, durability.
- Electrical safety and electromagnetic compatibility (EMC) reports (IEC 60601 series).
- Software documentation and testing (if applicable) per IEC 62304.
- Risk management file per ISO 14971.
- Usability engineering (IEC 62366).
- Biocompatibility testing for patient-contact materials (ISO 10993).
- Sterilization and packaging validation (if relevant).
- Labeling, instructions for use (IFU), and promotional materials draft.
- Quality system evidence (ISO 13485 or QSR compliance).
- Clinical data or literature summary supporting indications (if needed).
Engaging early with regulators and adopting international standards during design can reduce regulatory risk and speed time-to-market. ((https://www.longestmedical.com/regulatory-fda-clearance-shockwave-devices.html?utm_source=openai))
What Are the Market Considerations for Procuring Shockwave Therapy Devices?
When procuring shockwave therapy devices, consider the following:
- Device Type: Determine whether focused or radial shockwave therapy aligns with your clinical needs.
- Regulatory Status: Ensure the device has FDA clearance for the intended use.
- Clinical Evidence: Review published studies supporting the device's efficacy for specific indications.
- Cost and Return on Investment (ROI): Evaluate the device's cost relative to potential revenue generation and patient outcomes.
- Supplier Reputation: Assess the manufacturer's track record, customer support, and warranty offerings.
Conducting a thorough market analysis and consulting with industry experts can aid in making informed procurement decisions. ((https://www.longestmedical.com/b2b-buyers-guide-shockwave-therapy-machine.html?utm_source=openai))
How Does Longest Medical Support Medical Device Procurement?
Longest Medical offers comprehensive support for medical device procurement, including:
- Regulatory Guidance: Assistance with navigating FDA clearance processes and compliance with international standards.
- Product Selection: A curated portfolio of FDA-cleared shockwave therapy devices tailored to various clinical applications.
- Training and Support: Educational resources and ongoing support to ensure effective device utilization.
- Market Insights: Access to industry reports and analyses to inform procurement decisions.
Partnering with Longest Medical can streamline the procurement process and enhance clinical outcomes.
Conclusion
Selecting the appropriate FDA-cleared shockwave therapy device involves careful consideration of regulatory requirements, device specifications, clinical evidence, and market factors. Collaborating with experienced suppliers like Longest Medical can provide valuable support in making informed procurement decisions, ultimately leading to improved patient care and practice success.
References
- SoftWave Tissue Regeneration Technologies – June 1, 2021 – FDA clearance for OrthoGold 100 device.
- CuraMedix – Date not specified – FDA approval for DuoLith SD1 TTOP Ultra device.
- Zynex Medical – Date not specified – FDA clearance for TensWave device.
- InMode – Date not specified – Introduction of Evolve platform.
- Vale Medical – Date not specified – Distribution of Storz Medical devices.
- Longest Medical – Date not specified – Regulatory and market guidance for shockwave therapy devices.

World Diabetes Day: Longest Shockwave Therapy—New Hope for Diabetic Foot

World Stroke Day 2025: A Complete Guide from Prevention to Recovery

The Clinical Value of Longest LGT-2410S Cryotherapy Device: Mechanisms, Features, and Applications

Electrostatic Oscillation Therapy: Gentle Joint Pain Relief Guide

Longest Medical at MEDICA 2025: Visit Hall 4,G05 for Rehab Devices
LGT-231
Is OEM/ODM service available?
Yes. Contact us for more details. We offer professional OEM/ODM services.
LGT-2500S
Why should lincorporate ESWT into my clinic?
- 1. Proven Effectiveness: Many studies and cases have proven the effectiveness of ESWT in orthopedics, sports medicine, and musculoskeletal disorders.
2. Wide Range of Indications: ESWT can treat a wide range of indications, like shin splints, plantar fascitis, trigger points, jumper's knee, tennis elbow, hamstring pain, and much, much more. It can be used in many fields: physiotherapy, orthopedics, aesthetic medicine, sports medicine, and veterinary medicine.
3. Non-invasive, No Side Effects: No side effects reduce complaints and improve patients' satisfaction. Being non-invasive can avoid costly recovery time.
LGT-2320BE
Are there any side effects of using Electrical Stimulation Machine for aesthetic purposes?
Side effects are rare and minor. They are generally short-lived and resolve on their own. Consult a professional before treatment, especially if you have any concerns or underlying medical conditions.
LGT-2500S - Beauty
What is the product status of the LGT-2500S ?
The LGT-2500S is the first independently developed pneumatic ballistic extracorporeal shockwave with NMPA certification in China. It is also the first to obtain CE medical registration and be certified by and become a member of ISMST in China.
5- LGT-2500X copywriting, physical therapy version
What are the benefits of shockwave treatment?
The benefits of shockwave treatment include alleviating chronic pain, stimulating the body's natural healing response, promoting tissue regeneration, increasing blood circulation, and restoring mobility and functionality.

Professional 12-Channel Low-Frequency Electrical Stimulation Machine LGT-2320SP
LGT-2320SP is an advanced electrical stimulation sports training station. It leads to multisite high-efficiency strength gain and realizes bilateral balanced development with motor point detection.
Specifically designed for sports medicine professionals, this device is ideal for the treatment and rehabilitation of sports-related injuries. It delivers precise electrical impulses to targeted areas of the body, promoting pain relief, muscle strengthening, and accelerated recovery.

Professional 12-Channel Low-Frequency Electrical Stimulation Machine LGT-2320BE
The LGT-2320BE is an advanced EMS machine, leveraging the power of low-frequency electrical stimulation therapy to achieve remarkable results in body sculpting.
It not only efficiently contours and tones the body but also actively promotes muscle relaxation, relieving tension and soreness. Moreover, it plays a significant role in enhancing skin elasticity, rejuvenating the skin's appearance and suppleness.
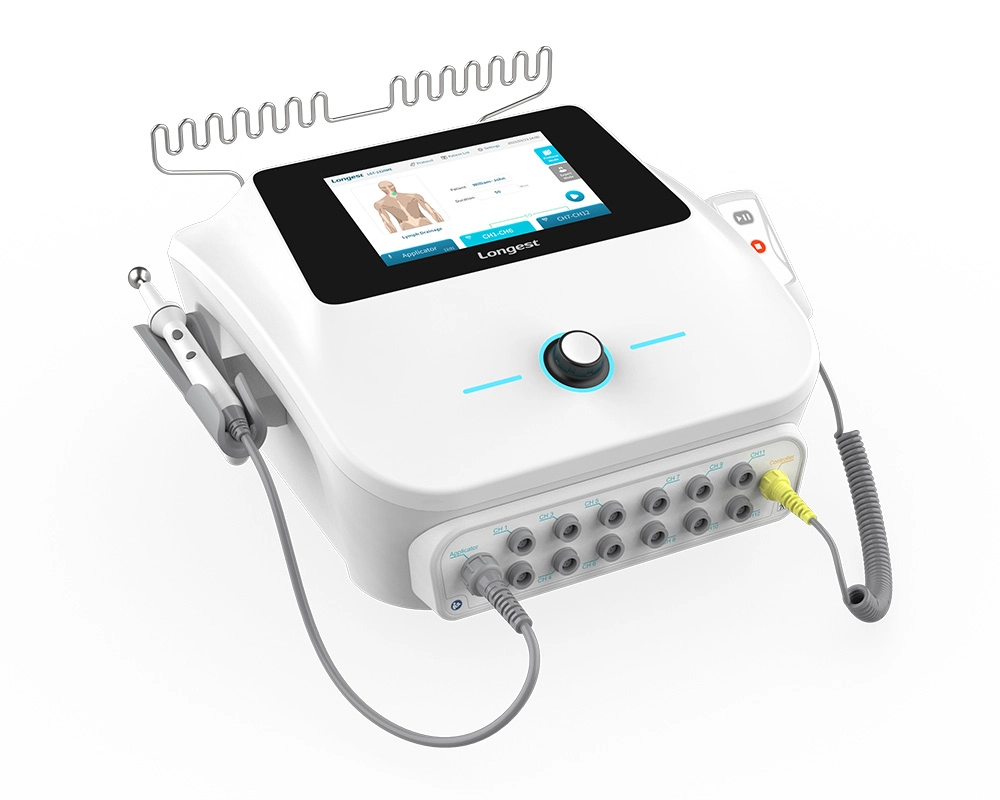
Neuromuscular Electrical Stimulation NMES Machine LGT-2320ME
The LGT-2320ME, a portable electro-stimulation therapy device, is mainly composed of the main unit, a hand switch, a pen electrode controller, and diverse types of electrodes.
It has the capacity to supply three channel groups (CH1-CH6, CH7-CH12, and the Applicator channel) with either TENS or NMES current. Specifically engineered for utilization in hospitals and clinics, this device effectively assists patients in reclaiming lost muscle strength and expedites recovery times, consequently enhancing the overall standard of patient care and bringing about more satisfactory rehabilitation outcomes.

Rehab Bike for Upper and Lower Limbs, Active-Passive Trainer (APT) RehaMoto LGT-5100D
RehaMoto controls the servo motor through the central processing unit and the biomechanical monitoring feedback system. Users can do passive, assisted, active, and constant-speed training by RehaMoto LGT-5100D Config I. The intelligent identification device realizes real - time monitoring of user training status and smooth conversion between different modes. Fully realize the best clinical training effect, and promote the recovery of users' motor function.
Get in touch with us
© 2025 Longest Medical. All Rights Reserved. Powered by gooeyun.






LongestMedical
LongestGloba
longest
guangzhou_longest
GzLongest