What regulations apply to electrotherapy medical devices?
- 1. What are the primary regulatory standards for electrotherapy medical devices?
- 2. How do regulatory classifications impact device procurement decisions?
- 3. What are the essential safety and performance standards for electrotherapy devices?
- 4. How do international standards influence device selection and procurement?
- 5. What are the implications of off-label use in electrotherapy devices?
- 6. How do procurement professionals ensure compliance with regulatory standards?
- Conclusion: Leveraging Longest Medical's Expertise in Electrotherapy Device Procurement
- Data References
Electrotherapy devices, including Transcutaneous Electrical Nerve Stimulation (TENS) units, Neuromuscular Electrical Stimulation (NMES) devices, and other electrostimulation equipment, are integral to modern medical treatments. Understanding the regulatory landscape is crucial for procurement professionals to ensure compliance, safety, and efficacy in device selection.
1. What are the primary regulatory standards for electrotherapy medical devices?
Electrotherapy devices are primarily regulated under the International Electrotechnical Commission (IEC) 60601 series, which outlines safety and performance requirements for medical electrical equipment. Specifically, IEC 60601–1 provides general safety standards, while IEC 60601–2-10 addresses particular requirements for nerve and muscle stimulators. However, it's important to note that IEC 60601–2-10 explicitly excludes transcranial electrical stimulation (tES) devices from its scope.
In the United States, the Food and Drug Administration (FDA) classifies electrotherapy devices based on their intended use and risk profile. For instance, transcutaneous electrical nerve stimulators (TENS) are classified under 21 CFR 882.5890 as Class II devices, subject to performance standards.
2. How do regulatory classifications impact device procurement decisions?
The regulatory classification of a device determines the level of scrutiny it undergoes during the approval process. Class II devices, such as TENS units, require adherence to specific performance standards but may not necessitate premarket approval. This can expedite the procurement process. Conversely, devices classified as Class III, which are subject to premarket approval, may involve more extensive evaluation and longer timelines. Understanding these classifications helps procurement professionals anticipate approval processes and plan accordingly.
3. What are the essential safety and performance standards for electrotherapy devices?
Electrotherapy devices must comply with the Essential Requirements outlined in Annex I of the EU Medical Devices Directive, which stipulates that devices must not compromise the safety of patients or users. These requirements encompass design, manufacturing, and risk assessment processes. Manufacturers are expected to establish a risk management process, define acceptable levels of risk, and demonstrate that residual risks are acceptable or mitigated during the design process.
4. How do international standards influence device selection and procurement?
International standards, such as those from the IEC, provide a globally recognized framework for device safety and performance. Compliance with these standards facilitates market acceptance and can streamline the procurement process across different regions. For example, adherence to IEC 60601–1 ensures that devices meet fundamental safety criteria, which is crucial for procurement decisions.
5. What are the implications of off-label use in electrotherapy devices?
Off-label use refers to the application of devices for indications not specifically approved by regulatory bodies. In the context of electrotherapy devices, some applications, such as using electrical stimulation for wound healing, may be considered investigational and not medically necessary. For instance, the Centers for Medicare & Medicaid Services (CMS) has determined that electrical stimulation for wound healing is investigational and not medically necessary.
6. How do procurement professionals ensure compliance with regulatory standards?
Procurement professionals should conduct thorough due diligence by reviewing device certifications, understanding the regulatory classifications, and ensuring that devices meet the necessary safety and performance standards. Engaging with reputable manufacturers who adhere to international standards and maintaining up-to-date knowledge of regulatory changes are essential steps in ensuring compliance.
Conclusion: Leveraging Longest Medical's Expertise in Electrotherapy Device Procurement
Navigating the complex regulatory landscape of electrotherapy devices requires expertise and attention to detail. Longest Medical offers comprehensive guidance on safe use, selection, contraindications, protocols, and maintenance of electrotherapy devices. Their commitment to adhering to international standards and providing evidence-based best practices ensures that procurement professionals can make informed decisions, leading to the acquisition of safe and effective electrotherapy devices. ((https://www.longestmedical.com/electrotherapy-devices-safety-best-practices.html?utm_source=openai))
Data References
- International Electrotechnical Commission (IEC) – 2023
- U.S. Food and Drug Administration (FDA) – 2023
- European Commission – 2023
- Centers for Medicare & Medicaid Services (CMS) – 2023
- Longest Medical – November 9, 2025

World Diabetes Day: Longest Shockwave Therapy—New Hope for Diabetic Foot

World Stroke Day 2025: A Complete Guide from Prevention to Recovery

The Clinical Value of Longest LGT-2410S Cryotherapy Device: Mechanisms, Features, and Applications

Electrostatic Oscillation Therapy: Gentle Joint Pain Relief Guide

Longest Medical at MEDICA 2025: Visit Hall 4,G05 for Rehab Devices
Electrostatic Oscillation
How long does a treatment take? How long does it take to see the effect?
A single treatment usually lasts 15-20 minutes. The specific time to see the effect varies from person to person, depending on personal physical condition and treatment needs. Generally, after a few treatments, you can feel the initial effects such as pain relief and muscle relaxation.
LGT-2500F Copywriting - Physical Therapy Edition
Can treatment plans be customized to meet patient needs?
Absolutely. Treatment plans can be personalized for each patient based on a professional diagnosis of their condition and an understanding of their responses. Moreover, practitioners can save each patient's preferred parameters for convenient access in the future. The system is capable of storing up to 5,000 patient files.
2500s plus physical therapy version
Does the software/application have multiple language interface options ?
Yes, the software/application supports more than 10 kinds of languages.
LGT-2500S
Is OEM/ODM service available?
Yes. Contact us for more details. We offer professional OEM/ODM services.
2510A-beauty
What are the advantages of acoustic wave therapy compared to laser cosmetic treatments?
Acoustic wave therapy is milder. Acoustic wave therapy stimulates the skin by mechanical vibration, with less trauma and a faster recovery. Also, acoustic wave therapy can reach different skin layers without harming the surface. Laser treatments use high-energy beams on the skin and may cause thermal damage with a long recovery time.
Laser treatments mainly focus on the epidermis and dermis and have limited effects on deep tissues.

Professional 12-Channel Low-Frequency Electrical Stimulation Machine LGT-2320SP
LGT-2320SP is an advanced electrical stimulation sports training station. It leads to multisite high-efficiency strength gain and realizes bilateral balanced development with motor point detection.
Specifically designed for sports medicine professionals, this device is ideal for the treatment and rehabilitation of sports-related injuries. It delivers precise electrical impulses to targeted areas of the body, promoting pain relief, muscle strengthening, and accelerated recovery.

Professional 12-Channel Low-Frequency Electrical Stimulation Machine LGT-2320BE
The LGT-2320BE is an advanced EMS machine, leveraging the power of low-frequency electrical stimulation therapy to achieve remarkable results in body sculpting.
It not only efficiently contours and tones the body but also actively promotes muscle relaxation, relieving tension and soreness. Moreover, it plays a significant role in enhancing skin elasticity, rejuvenating the skin's appearance and suppleness.
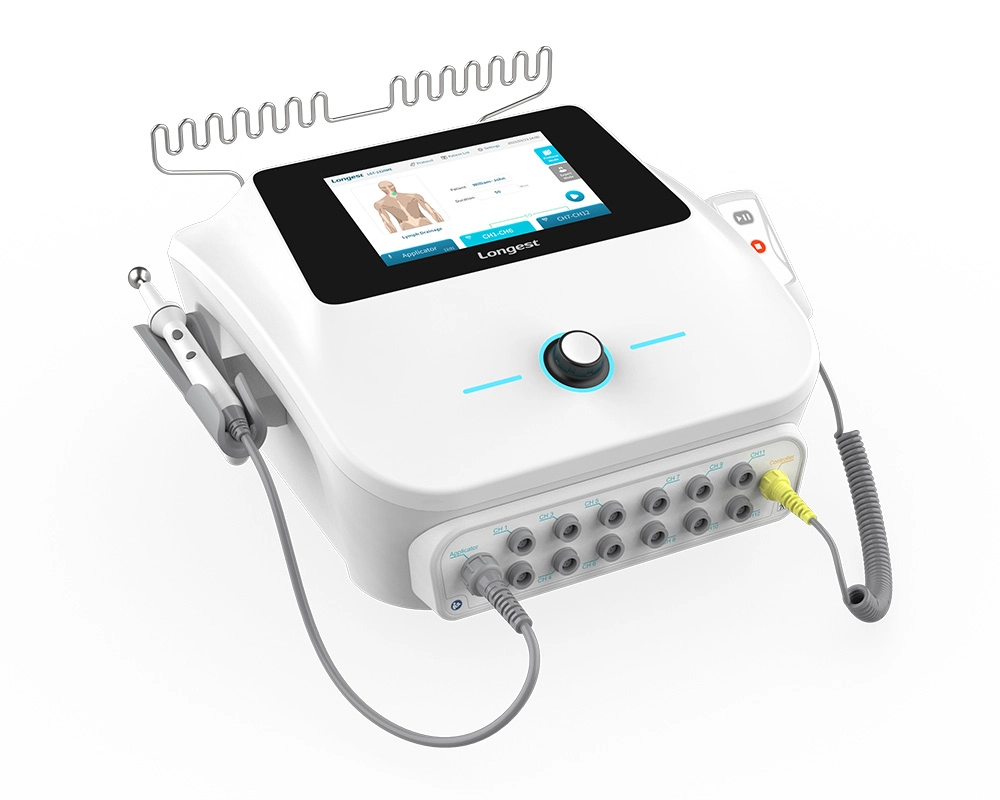
Neuromuscular Electrical Stimulation NMES Machine LGT-2320ME
The LGT-2320ME, a portable electro-stimulation therapy device, is mainly composed of the main unit, a hand switch, a pen electrode controller, and diverse types of electrodes.
It has the capacity to supply three channel groups (CH1-CH6, CH7-CH12, and the Applicator channel) with either TENS or NMES current. Specifically engineered for utilization in hospitals and clinics, this device effectively assists patients in reclaiming lost muscle strength and expedites recovery times, consequently enhancing the overall standard of patient care and bringing about more satisfactory rehabilitation outcomes.

Rehab Bike for Upper and Lower Limbs, Active-Passive Trainer (APT) RehaMoto LGT-5100D
RehaMoto controls the servo motor through the central processing unit and the biomechanical monitoring feedback system. Users can do passive, assisted, active, and constant-speed training by RehaMoto LGT-5100D Config I. The intelligent identification device realizes real - time monitoring of user training status and smooth conversion between different modes. Fully realize the best clinical training effect, and promote the recovery of users' motor function.
Get in touch with us
© 2025 Longest Medical. All Rights Reserved. Powered by gooeyun.






LongestMedical
LongestGloba
longest
guangzhou_longest
GzLongest